Author, year
Trial name
Laparoscopic patients
Open patients
Conversion rate (%)
Positive margin RR
Complications
Hospital stay
Guillou 2005 [3]
MRC CLASSIC triala
253
128
32.40
1.08 (0.60–1.97)
1.08 (0.83–1.43)
Favored laparoscopic surgery
Kang 2010 [4]
COREAN trial
170
170
1.20
0.71 (0.23–2.21)
0.90 (0.61–1.34)
No difference
van der Pas 2013 [5]
COLOR II trial
699
345
17
0.95 (0.63–1.45)
1.08 (0.91–1.27)
Favored laparoscopic surgery
In addition to the major trials listed above, a recently published Cochrane review [6] assessed 11 large and small trials involving 3812 patients. The Cochrane group found no differences in rates of positive CRM, 30-day morbidity, 30-day mortality, or number of lymph nodes retrieved. Patients undergoing laparoscopic surgery had a decreased risk of wound infection (OR 0.68, 95% CI 0.50–0.93, P = 0.015), decreased use of analgesia (−0.60 doses, 95% CI −0.93 to −0.27, P < 0.001), decreased length of stay (−2.16 days, 95% CI −3.22 to −1.10, P < 0.001), decreased time to diet (−0.53 days, 95% CI −0.80 to −0.23, P < 0.001), and decreased time to defecation (−0.86 days, 95% CI −1.17 to −0.54, P < 0.001), compared to patients undergoing open surgery.
Long-Term Outcomes of Laparoscopic Rectal Cancer Surgery
Two of the large rectal cancer trials (the MRC CLASICC trial and the COREAN trial) [3, 7] have reported long-term outcomes comparing laparoscopic to open surgery. The results are summarized in Table 10.2. No differences between laparoscopic and open surgery have yet been observed with respect to the risk of local recurrence, distant metastasis, or overall survival. However, both the COLOR II and ACOSOG Z6051 trials have yet to publish long-term outcomes at the time of preparation of this summary.
Table 10.2
Long-term outcomes from RCT assessing laparoscopic vs. open surgery
3-year disease-free survival | 3-year overall survival | 3-year local recurrence | ||||
|---|---|---|---|---|---|---|
Laparoscopic | Open | Laparoscopic | Open | Laparoscopic | Open | |
COREAN Trial | 79.2% | 72.5% | 88.0% | 85.0% | 2.6% | 4.9% |
MRC CLASICC Trial | 66.3% | 67.7% | 68.4% | 66.7% | 7.9% | 8.6% |
A Cochrane review of both colon and rectal cancer patients found no differences in 5-year overall survival (OR 1.15, 95% CI 0.87–1.52, P = 0.32), 5-year local recurrence (OR 0.94, 95% CI 0.49–1.81, P = 0.32), distant recurrence (OR 0.97, 95% CI 0.70–1.32, P = 0.80), or port site/wound recurrence (2.76, 95% CI 0.75–10.20, P = 0.13) [8] between open and laparoscopic surgery. Thus, the currently available data appear to support minimally invasive surgery for rectal cancer, reporting short-term benefits similar to those seen in colon cancer.
Trends in Minimally Invasive Surgery
In light of the observed short-term benefits of minimally invasive surgery compared to open surgery—as well as increased technical expertise on the part of clinicians and improvement in technique and devices—the use of laparoscopic colon surgery has increased dramatically during the past two decades. Kang et al. compared the frequency of laparoscopic surgery from 2007 to 2009 using the National Inpatient Sample. They found that the proportion of patients undergoing a laparoscopic right colectomy increased from 13% in 2007 to 49% in 2009 [7]. A large increase was also seen in laparoscopic sigmoid resection (18% in 2007 to 55% in 2009). In addition, the rates of conversion in sigmoid resection dropped dramatically between 2007 and 2009, indicating significant advances along the surgical learning curve, as well as improved instrumentation. However, during this time there was very little increase in the use of laparoscopic surgery for low anterior resection of rectal cancer (12% in 2007 to 16% in 2009) [9] (Figs. 10.1 and 10.2).
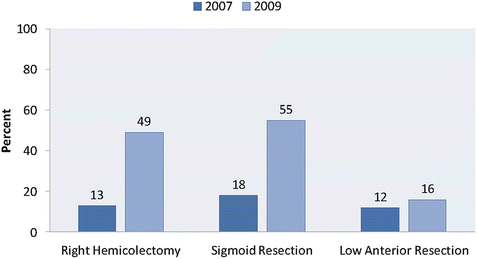
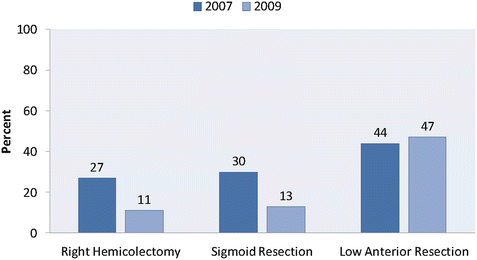

Fig. 10.1
Proportion of surgeries performed laparoscopically. Proportion of colon and rectal resections (right hemicolectomy, sigmoid resection, low anterior resection) performed laparoscopically from 2007 to 2009. (Adapted from Kang CY, Halabi WJ, Luo R, Pigazzi A, Nguyen NT, Stamos MJ. Laparoscopic colorectal surgery: a better look into the latest trends. Arch Surg. 2012;147(8):724–31)

Fig. 10.2
Conversion from laparoscopic to open surgery. Proportion of laparoscopic colon and rectal resections (right hemicolectomy, sigmoid resection, low anterior resection) requiring intraoperative conversion from 2007 to 2009. (Adapted from Kang CY, Halabi WJ, Luo R, Pigazzi A, Nguyen NT, Stamos MJ. Laparoscopic colorectal surgery: a better look into the latest trends. Arch Surg. 2012;147(8):724–31)
The difficulty in adapting laparoscopic techniques to rectal cancer surgery stems from the anatomical restrictions imposed by the operative field and the technical demands of these procedures. The narrow, bony pelvis can inhibit proper visualization of anatomical landmarks and restrict surgical movement; furthermore, it is hardly an ideal space for the straight, rigid instruments used in traditional laparoscopy. The limitations inherent in maneuvering laparoscopic instruments and the difficulty in achieving proper retraction are a challenge. The proximity of other pelvic organs adds a layer of difficulty not encountered in segmental colon resection. In addition, there is the potential for collision of instruments within the small pelvic working space. In rectal cancer surgery, the need for complete and total mesorectal excision (TME) , entailing exact and sharp dissection of the mesorectal fascia, is paramount. Achieving nerve preservation in order to maintain the patient’s postoperative bladder and sexual function, whenever possible, is also an important goal.
Many of the limitations of laparoscopic pelvic dissection can be eliminated when using the robotic surgical platform. The robot’s articulating instruments facilitate dissection within the difficult confines of the pelvis. A stable camera platform, and the ability of the surgeon to control camera location, provides greatly enhanced 3-D visualization of the operative field—far superior to the two-dimensional visuals provided by the laparoscope. Retraction with the third robotic arm—so difficult to achieve with traditional laparoscopic instruments—can be controlled directly by the operating surgeon without impingement on the working space. In addition, the articulating instruments decrease the risk of collision.
Laparoscopic vs. Robotic Surgery for Rectal Cancer: A Review of the Literature
The safety and efficacy of robotic rectal surgery has been assessed in a number of observational studies as well as in several small randomized, controlled trials. The largest trial, robotic vs. laparoscopic resection for rectal cancer (ROLARR trial) , is currently open to accrual and anticipates enrolling 400 rectal cancer patients. The primary outcome will be conversion to open surgery. Secondary outcomes include circumferential radial margin and other margin positivity. The investigators will also examine morbidity and mortality, 3-year disease-free survival, and postoperative sexual function [10].
Currently, several meta-analyses have been completed [11–13]. The findings are summarized in Table 10.3. These studies consistently reported a lower risk of conversion to open surgery in the robotic group (OR 0.26, 95%CI 0.12–0.57) (Fig. 10.3). A meta-analysis of randomized, controlled trials [14] focused on four studies comparing robotic to laparoscopic surgery in colorectal cancer. The conversion rate was found to be lower in the robotic group (OR 0.26, 95% CI 0.12–0.57) (Fig. 10.4). There was no difference in length of stay, rate of complications, or number of harvested lymph nodes. A large population-based study [9] also compared rates of conversion between laparoscopic and robotic surgery, with similar findings (Fig. 10.5). Unlike laparoscopic rectal resection, patient obesity and low-lying tumors are not strongly associated with conversion in robotic surgery [15, 16].
Table 10.3
Findings from meta-analyses comparing robotic to laparoscopic surgery
Author | Number of studies | Blood loss | Operative times | Conversion to open surgery | Complications | Length of stay | Cost | Lymph node yield | CRM |
|---|---|---|---|---|---|---|---|---|---|
Yang et al. | 16 | Robotics | Laparoscopic | Robotics | No difference | No difference | Laparoscopic | No difference | No difference |
Trastulli et al. | 8 | No difference | Robotics | No difference | No difference | No difference | No difference | ||
Memon et al. | 7 | No difference | Robotics | No difference | No difference | No difference | No difference | ||
Lin et al. | 8 | No difference | No difference | Robotics | No difference | No difference | No difference | No difference | |
Xiong et al. | 8 | No difference
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|