Fig. 18.1
Historic progression toward the current standard of care for locally advanced rectal cancer: chemoradiotherapy before total mesorectal excision. XRT radiotherapy, CRT chemoradiation therapy, TME total mesorectal excision, LR local recurrence, DR distant recurrence, OS overall survival
Radiosensitizing Agents
Numerous chemotherapeutic agents—most of which have shown some radiosensitizing effects in preclinical studies—have been tested in the neoadjuvant setting to accompany radiotherapy (Table 18.1). Some, like irinotecan, have been tested in LARC primarily because of its observed benefit in metastatic colorectal cancer. With the exception of fluoropyrimidines, however, none have been effectively validated in prospective trials.
Table 18.1
Chemotherapeutic agents considered and explored as radiosensitizers or for total neoadjuvant therapy approaches
Agents | Mechanism | Common side effects |
|---|---|---|
5-FU | Pyrimidine analog, thymidylate synthase (TS) inhibitor | GI, mucositis |
Folinic acid (leucovorin) | Enhances 5-FU effects and contributes to TS inhibition | – |
Irinotecan | Topoisomerase I inhibitor | GI, leukopenia |
Capecitabine | Prodrug of 5-FU | GI, mucositis |
Oxaliplatin | Platinum-based agent, DNA cross-linking | Peripheral neuropathy, GI |
Cetuximab/panitumumab | Anti-EGFR monoclonal antibody (for use with KRAS wild-type tumors only) | Skin rash |
Bevacizumab | Anti-VEGF monoclonal antibody | Compromised wound healinga |
Fluoropyrimidines
5-Fluorouracil (5-FU) is the primary chemotherapeutic agent used in the radiosensitization in rectal cancer. While its potential to create a state of radiosensitivity was recognized early on, studies eventually led to the understanding that the benefit of 5-FU was inextricably linked to the schedule of its administration. 5-FU must remain present after radiation exposure to establish and maintain the radiosensitive state; for this reason, bolus 5-FU quickly fell out of favor, and continuous venous infusion (CVI) of 5-FU 225 mg/m2 daily became the standard [22].
The overall benefit of combining chemotherapy with radiation therapy (RT) was proven by several randomized trials by the North Central Cancer Treatment Group (NCCTG) and the Gastrointestinal Tumor Study Group (GITSG) that compared postoperative radiotherapy alone versus radiotherapy with 5-FU administered either as a bolus or by CVI [23, 24]. From other studies that followed in the NCCTG and the Intergroup consortia, it became apparent that CVI 5-FU was associated with lower hematologic toxicity and longer overall survival compared to bolus 5-FU [25, 26]. The European Organization for Research and Treatment of Cancer (EORTC) protocol 22921 was developed to assess the effect of adding chemotherapy to preoperative RT and the value of postoperative chemotherapy in LARC [11, 27]. They randomized 1011 patients across four arms: (a) preoperative radiotherapy, (b) preoperative radiotherapy plus bolus 5-FU and leucovorin, (c) preoperative radiotherapy followed by postoperative chemotherapy, and (d) preoperative radiotherapy and bolus 5-FU and leucovorin followed by postoperative chemotherapy. Five-year local recurrence was significantly lower in all three arms receiving any form of chemotherapy (pre- or postoperative) compared to radiotherapy alone, though survival was not significantly altered. More recently, in a randomized phase III trial, Hofheinz and colleagues showed non-inferiority of capecitabine, the oral prodrug of fluorouracil, when compared to 5-FU. This provided a convenient treatment alternative for reliable and motivated patients [28]. The equivalence of capecitabine and 5-FU has also been corroborated with the NSABP-R04 cohort [29].
Oxaliplatin
A number of large phase III trials have evaluated the potential role of oxaliplatin in increasing radiation sensitivity. The STAR-01 [30], the ACCORD 12/0405-PRODIGE2 [31], and the NSABP-R04 [29] trial each investigated the addition of oxaliplatin to a fluoropyrimidine. This combination, however, appeared to result in greater toxicity with no improvement in response or therapeutic benefit. Conversely, the CAO/ARO/AIO-04 trial [32] found that the inclusion of oxaliplatin to a 5-FU-based CRT regimen led to a higher pathologic complete response (pCR) rate, with no increase in toxicity. While encouraging, their 5-FU dosing and schedule differed between the control arm and the arm with oxaliplatin, which may have affected the outcomes. At this point, oxaliplatin is not routinely included in the neoadjuvant regimens currently used for rectal cancer. Together, these four trials have also demonstrated that neoadjuvant chemoradiation using a fluoropyrimidine-based chemosensitization agent achieves a pCR rate between 13% and 19%.
EGFR Inhibitors
The success and efficacy of anti-EGFR agents like cetuximab, panitumumab, and nimotuzumab in KRAS wild-type metastatic colorectal cancer prompted a number of studies evaluating its use in the preoperative treatment of LARC. Response rates with the addition of EGFR inhibitors to 5-FU and radiotherapy have been inconsistent; increased response is seen in some studies [33], but most studies have demonstrated equivocal results [34, 35]. The EXPERT-C trial randomized 165 patients to four cycles of capecitabine/oxaliplatin before chemoradiotherapy, with or without weekly cetuximab during the CAPOX and CRT regimens. While cetuximab was shown to have an increase in radiologic response and overall survival in KRAS/BRAF wild-type rectal cancers, the primary endpoint of improved pCR was not met [36]. Some studies have reported a worse response, which suggests there may be mechanisms of response in tumors to these combined modality treatments that are not yet understood [37]. In a review of multiple phase I/II trials that included cetuximab in the neoadjuvant regimen, Glynne-Jones et al. measured a pooled pCR for cetuximab-based regimens across 11 studies at 10.71%, compared with a pCR rate of around 13% with other standard fluoropyrimidine-based CRT schedules [37]. Given these findings, EGFR inhibitors are not used in the neoadjuvant setting as a radiosensitizer at this time.
Irinotecan
This topoisomerase inhibitor has shown significant antitumor activity in metastatic colorectal cancer. While there have been small phase II trials showing that irinotecan may be effective and safe as an adjunct to traditional 5-FU and radiotherapy [38, 39], there have not been any phase III trials assessing its relative efficacy compared to 5-FU and radiotherapy alone.
Other Agents
Urick and colleagues reported enhanced radiosensitization when MEK inhibition by selumetinib was added in both in vitro settings and in vivo HCT116-derived xenografts [40]. Kleiman and colleagues focused on KRAS mutant rectal cancers that are widely reported to be more resistant to CRT and tested multiple small molecular inhibitors for potential radiosensitizing capability. They found a Chk1/2 inhibitor and a PI3K/mTOR inhibitor to be particularly promising, as these had a synergistic effect combined with 5-FU [41]. Other experimental approaches have attempted to increase radiation sensitivity by imposing additional oxidative stress with less toxic compounds such as zerumbone, a compound derived from ginger [42]. Many of these experimental approaches appear promising but have not yet been validated in clinical trials. Conceivably, they could help improve local control and increase the rate of pCR.
Short- Versus Long-Course Radiotherapy
The efficacy of RT in the setting of high-quality surgical resection using TME principles was specifically explored in the Dutch Colorectal Cancer Group trial mentioned previously, which randomized 1861 patients with resectable disease to TME alone or to short-course RT (SCRT) followed in 2–7 days by TME. They found patients in the RT arm had a reduced rate of local recurrence compared to TME alone; however, SCRT did not affect survival [9]. This study effectively demonstrated the value of RT, however, lending early support for a short-course modality.
In SCRT , 25 Gy is usually provided in five 5 Gy fractions, compared with long-course radiation, which generally delivers 1.8 Gy across 28 fractions, along with the concurrent use of radiosensitizing 5-FU. Proponents of the SCRT highlight patient convenience and lower costs as important advantages, while those supporting long-course radiation point at decreased surgical morbidity and improved sphincter preservation, along with the benefits of chemosensitizing agents (which cannot be given safely simultaneously in the SCRT regimen). In addition, some studies have reported that the higher dose per fraction (5 Gy compared with 1.8 Gy) increases the risk of delayed toxicity and that tumor regression is lower with SCRT [9, 43]. Few trials have sought to compare these two approaches directly, though.
Bujko and colleagues are the only group to have prospectively compared the two, randomizing 316 patients to either CRT or SCRT, and found that long course was associated with a significantly decreased incidence of positive radial margins (4.4% vs. 12.9%, p = 0.017) and a higher rate of pCR (0.7% vs. 16.1%); however, this did not carry over into a significant difference in local recurrence or survival [44]. Moreover, they reported greater radiation toxicity in the CRT group and poorer compliance to treatment schedule. Their conclusion was that SCRT was a viable alternative to CRT with neither holding a long-term oncologic advantage, but SCRT is potentially beneficial in terms of lowering cost, and lower morbidity is associated with its use. More recently, the Trans-Tasman Radiation Oncology Group 01.04 randomized 326 patients with ERUS- or MRI-staged T3,N0–2,M0 tumors to SCRT and surgery followed by 6 months of adjuvant chemotherapy or CRT and surgery followed by 4 months of adjuvant chemotherapy [45]. Their study was powered to detect a 10% difference in local recurrence at 3 years, with a 5% level of significance. They reported a trend toward lower 5-year cumulative incidences of local recurrence for long-course CRT compared with SCRT (5.7% vs. 7.5%, p = 0.51). However, the differences were not statistically significant. Moreover, patient imbalances between groups have been called to attention, with fewer patients with low rectal cancers in the CRT than the SCRT arms, and varying rates of APR. The quality of surgery and accuracy of MRI staging have also been criticized [46, 47].
The CRT-course divide has, therefore, remained somewhat static across national boundaries, with a Western preference for long-course CRT and a majority of European countries favoring SCRT. Because of a current trend exploring the incorporation of therapies traditionally reserved for the adjuvant period into the neoadjuvant regimen, combinations of either short-course RT or long-course CRT with systemic therapies are being explored and gaining greater traction. As such, it may become increasingly difficult to determine whether short-course or long-course RT is more effective as independent neoadjuvant modalities.
Time Interval between CRT and Surgery
Apart from sporadic observations and reports between the 1960s and 1990s, not much attention has been placed on the interval between CRT and surgery [48]. The reasons for this are probably multifaceted, but the arbitrary 6–8-week interval between the end of CRT and surgery has spread widely and is still maintained today. Nevertheless, in the 1990s, Brierley and colleagues from the Princess Margaret Hospital used radiation alone for 229 patients whose tumors were deemed unresectable or who were medically unfit for surgery or declined to have surgery [49]. They made a key observation that out of 66 patients who had a clinical complete response (cCR) to radiation, approximately 60% had tumor involution at 4 months and the remainder at 8 months, far longer than the standard 6–8 weeks. This suggested that the extent of radiation-induced tumor necrosis would have continued beyond the time of surgery. The reluctance to extend the duration is partly due to the well-recognized increase in fibrosis following completion of RT, which increases the difficulty of the operation (although it has not been measured systematically). At least one retrospective study reported increased morbidity and worse outcomes associated with a delay in surgery [50]. However, this has yet to be verified in any prospective cohort. In fact, many retrospective studies since then have found that a prolonged interval between CRT and surgery is associated with a greater tumor response [51–54].
In the Lyon R90-01 trial —one of the only prospective trials assessing the impact of the CRT-to-surgery interval—Francois and colleagues [55] randomized patients to a long interval (6–8 weeks) and short interval (<2 weeks) and found that the longer interval was associated with a higher rate of complete response (26% vs. 10.3%, p = 0.005), without a significant increase in surgical morbidity. More recently, Habr-Gama and colleagues evaluated a retrospective cohort of 255 patients [56], splitting the cohort into two groups, >12-week CRT-to-surgery interval and <12-week CRT-to-surgery interval; they also found that a longer interval increased the response rate, without increasing surgical morbidity.
The Timing of Rectal Cancer Response to Chemoradiation Consortium recently completed their study [57], which consisted of a series of prospective study groups measuring the rate of pCR when increasing the CRT-to-surgery interval with the addition of cycles of mFOLFOX-6 in the neoadjuvant setting. They achieved a high pCR rate of 38% by extending the CRT-to-surgery interval of 19.3 weeks, with the incorporation of six cycles of mFOLFOX-6 following chemoradiation in the last of their study groups. While their study cannot delineate whether the increased rate of response was due to the prolonged interval or the addition of up to six cycles of mFOLFOX-6 to the regimen, it does demonstrate that the prolonged interval does not increase surgery-related morbidity or operative difficulty.
Incorporation of Traditional Postoperative Systemic Chemotherapies into the Neoadjuvant Setting
The aforementioned optimization of CRT and surgery results in excellent local control of LARC, but the proportion of patients developing distant metastasis after a seemingly curative resection remains high. Understandably, this has shifted the focus of many studies toward improved control of distant metastasis, which is now the major cause of long-term mortality from this disease. Most clinicians have based adjuvant chemotherapy for patients with LARC on the lessons learned from managing colon cancer. Another approach also extrapolates the benefits of systemic chemotherapy for colon cancer but has led to explorations on the impact of shifting traditional adjuvant chemotherapies to the neoadjuvant, preoperative setting, either before chemoradiation (induction chemotherapy) or after chemoradiation (consolidation chemotherapy). Certain groups have dubbed these efforts complete or total neoadjuvant therapy (TNT). These studies have been performed most commonly with fluoropyrimidine-/oxaliplatin-based regimens, but other targeted agents such as those summarized in Table 18.1 have been tested as well. The rationale behind these approaches is to introduce systemic chemotherapy early, theoretically acting upon micrometastatic disease and potentially reducing eventual distant recurrence. Tumors may also be more responsive to chemotherapy delivered using their original blood supply, compared to the disrupted supply after surgery [58]. Beyond these theoretical advantages, the TNT approach is a practical response to the observation that nearly one-third of patients eligible for adjuvant chemotherapy never receive it and more than half never complete it as planned [11, 32]. Although almost all patients who receive CRT and TME should undergo adjuvant therapy, many forgo adjuvant chemotherapy due to postoperative complications, the presence of an ostomy, delays in ostomy reversal [59], or outright refusal [60]. More to the point, poor compliance to adjuvant therapy is associated with worsened survival in CRC. A systematic review of 10 studies including more than 15,000 patients demonstrated that each 4-week delay in the initiation of adjuvant chemotherapy corresponded to a 14% decrease in overall survival [61], a worrisome observation that could probably be generalized to rectal cancers. Interestingly, a recent systematic review and meta-analysis of four large phase III European trials disputed these results, showing no difference in overall survival whether adjuvant chemotherapy was given or not [62]. At this point, adjuvant chemotherapy following CRT and TME is still the standard of care. However, in light of the questions regarding its efficacy, alternative sequencing might be more accepted. Providing all or part of these therapies in the neoadjuvant setting bypasses many of these issues.
Induction Chemotherapy
Various regimens of neoadjuvant chemotherapy given prior to CRT have been tested in phase I and II trials, and most have delivered promising results, demonstrating improved tumor response rates and some suggestion of improved oncologic outcomes, with acceptable toxicity [63, 64]. Some of the earlier cohorts focused on patients thought to be at especially high risk based on imaging or clinical characteristics [65]. With encouraging response rates from these cohorts, other trials have extended these findings to regular LARC groups. One of the more tested induction regimens consists of capecitabine and oxaliplatin (CAPOX) prior to CRT.
A trial led by Maréchal and colleagues randomized patients to standard CRT and surgery or FOLFOX followed by CRT and surgery; they did not identify any improvement in tumor response, and the trial was closed due to futility. It did, however, demonstrate that the regimen was tolerable [66]. Cercek and colleagues from Memorial Sloan Kettering Cancer Center recently reviewed their experience using FOLFOX prior to CRT, demonstrating excellent treatment compliance and no signs of serious adverse effects [67]. All patients treated in their cohort underwent TME with an R0 resection, and nearly half had a tumor response greater than 90%. Even more impressive was the fact that 36% achieved either pCR or cCR, with a carefully selected group of clinical complete responders not undergoing TME at all.
Several groups have also been tested using targeted therapy as part of the induction regimen, such as VEGF inhibitor bevacizumab [68]. The EXPERT-C trial [36] tested an induction CAPOX regimen, with or without cetuximab. In the latter, Dewdney and colleagues show that KRAS/BRAF wild-type tumors (which are those expected to respond to the additional EGFR inhibitor) had greater radiologic response and overall survival, but not a higher rate of complete response after a median follow-up of close to 3 years [36].
Consolidation Chemotherapy
In the recently completed Timing of Rectal Cancer Response to Chemoradiation trial , we found that delivering two, four, or six cycles of mFOLFOX-6 after standard CRT in patients with LARC increased pCR rates progressively up to 38%, compared with a baseline 18% with CRT alone. No increase in adverse events, surgical complications, or risk of progression was observed [36]. Along similar lines, Hong and colleagues showed that FOLFOX consolidation improves 3-year DFS compared with 5-FU+LV following CRT and TME [69]. While completely theoretical and unproven, it is possible that providing chemotherapy after CRT may result in higher rates of pCR because the additional chemotherapy extends the duration between radiation and surgery, providing added time for the tumors to respond. Habr-Gama and colleagues have also studied consolidation therapy using three cycles of bolus 5-FU over 9 weeks following CRT; they have also noted a higher rate of pCR, with no increase in postoperative complications [70].
The Rectal Cancer and Preoperative Induction Therapy Followed by Dedicated Operation (RAPIDO) trial is currently randomizing patients to preoperative short-course radiation followed by six cycles of CAPOX and TME versus traditional preoperative long-course CRT and TME, measuring 3-year disease-free survival as their primary outcome [71].
Induction vs. Consolidation
The cumulative experience leads us to ask whether one sequence (induction or consolidation) is better than the other. A new prospective trial at MSK (NCT02008656) is testing the hypothesis that TNT will improve 3-year disease-free survival compared to standard CRT. In this trial, patients are being randomized to induction or consolidation therapy with FOLFOX, which should provide data to compare these two approaches.
There is no reason to assume that one approach will prove better than the other or that an entirely new sequence might not be found. For example, a small phase II trial by Gao and colleagues recently reported one of the highest pCR rates published for LARC thus far, with 19 of 45 (42.2%) patients achieving pCR and another 40% achieving near-complete response. They described their regimen as a “sandwich,” with one cycle of induction XELOX, followed by CRT and then followed by another cycle of consolidation XELOX before surgery in 6–8 weeks [72].
It can also be speculated that additional neoadjuvant therapy selects out certain aggressive and resistant tumors that are likely to progress to metastatic disease regardless of the current treatment approach. For these tumors, the administration of additional neoadjuvant therapy potentially provides the opportunity to identify distant progression, which might spare these patients radical surgery that would not benefit them and might be harmful. It is imperative to highlight the exploratory nature of these regimens, and a main concern which must be addressed and measured is whether these intensive neoadjuvant regimens increase surgical morbidity or overall toxicity. Most of these trials have collected data that is not fully mature, with no long-term oncologic and survival data. Nevertheless, given consistent improvement in tumor response and the increased proportions of pCR (which serves well as a surrogate marker), it is anticipated that we will see measurable gains in the coming years. It seems safe to say that these intensive neoadjuvant regimens will play an important role in the treatment of LARC over the coming decade.
Assessment of Response
Taking advantage of tumor response obviously requires accurate recognition of response. One of the challenges we face when trying to maximize neoadjuvant therapy is our ability to accurately assess tumor response to those therapies. When a complete or near-complete response is achieved after neoadjuvant therapy, we are increasingly inclined to consider either a local excision procedure or a “watch-and-wait” approach (Fig. 18.2). In doing so, however, we forgo the opportunity to thoroughly assess tumor response. In the case of local excision, we forgo the pathologic assessment of nodal disease, and in “watch-and-wait” approaches , we forgo the assessment of both the tumor and locoregional nodes altogether. Since the standard algorithms utilize pathological information to decide upon adjuvant therapy, our inability to fully assess the tumor may compromise our decisions regarding adjuvant treatment. What we are left with is the clinical or radiologic assessment of response. Although imperfect, these assessments are also improving. Our institution has settled upon a schema to assess tumor response in an attempt to standardize assessments that have potentially been heterogeneous (Fig. 18.3).
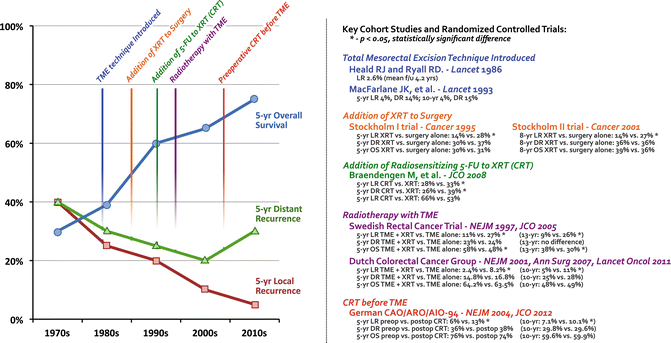
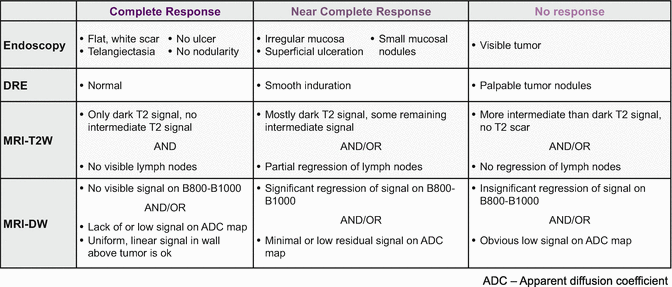

Fig. 18.2
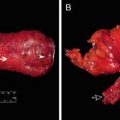
cT3N2 rectal cancer before therapy (top) and after therapy consisting of eight cycles of FOLFOX and 50.4 Gy of chemoradiation (bottom), demonstrating a clinical complete response and currently managed with a “watch-and-wait” strategy

Fig. 18.3
Schema for classifying tumor response to neoadjuvant therapy
It has been noted that digital rectal examination after CRT, even when performed by experienced colorectal surgeons, is unreliable in determining pCR [73]. While similar studies using ERUS and ERUS/CT to assess response after CRT seemed to show reasonable correlation with subsequent pathologic staging, the accuracy still ranged from 40% to 75% [74, 75]. Across each of these studies, overstaging of the residual tumor was more common than understaging. This suggests that patients are less likely to be undertreated if we relied solely upon these measures, but more accurate measures are still needed. Part of this is because clinical assessment of tumor response has typically been performed early on after neoadjuvant treatment, when tumors have not responded completely and when the pCR rate was low upon subsequent surgical resection. If the pCR rate is increased through an intensified neoadjuvant approach, we would expect that complete response would become easier to identify in the clinical setting.
A growing body of research has found that both 18FDG-PET/CT [76–79] and multiparametric MRI [80–83] can be used to distinguish between responders and nonresponders by comparing pre- and post-CRT imaging features . These have generally shown strong concordance with subsequent pathologic assessment of pCR and tumor regression [84, 85]. The goal is to improve post-CRT radiologic assessment of response to more closely approximate pathologic assessments. If pathologic response can be more accurately predicted, we will have greater confidence in offering patients with responsive tumors alternative approaches to traditional treatment algorithms.
Some small exploratory studies have assessed radiologic response to CRT very early in the course of CRT. Using 18FDG-PET/CT, Goldberg et al. performed a small prospective study of 20 patients and found that >32% decrease in maximum SUV was predictive of pCR after 1 week of treatment [86]. Similarly, a pilot study at MSK using DWI/DCE-MRI at early time points during CRT to evaluate their potential use in predicting response is currently under way (ClinicalTrials.gov Identifier: NCT01830582).
Setting the Right Limits
Chemoradiation has clearly proved itself invaluable at controlling local recurrence of rectal cancer. The weight of evidence also demonstrates that systemic chemotherapy—when applied in the neoadjuvant setting—similarly controls tumor progression, possibly acting on micrometastatic disease to improve distant control. But while the benefits of these intensive neoadjuvant regimens are alluring, they have also sparked a heated debate about whether all patients require such intensive treatment. The oncologic success in treating LARC has been achieved at the cost of significant morbidity and compromised quality of life [43, 59, 87, 88]. The task before us is to develop treatment approaches that maximize oncologic outcome while preserving quality of life by minimizing the morbidity associated with any treatments (Fig. 18.4) [89]. Do all patients with LARC require CRT, chemotherapy, and TME? The necessity of such an intense multimodality approach should naturally be questioned.
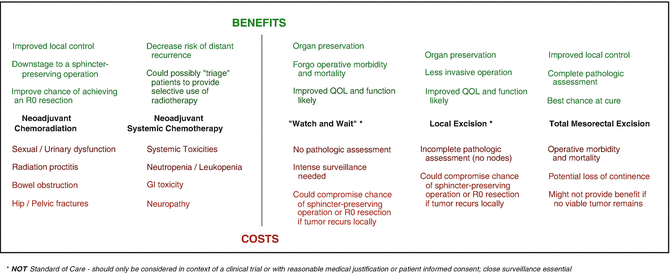

Fig. 18.4
Balancing the benefits and costs of multimodality therapy and less invasive surgical strategies
In a number of European countries, the “right limits” have been framed around MRI-based measures of tumor aggressiveness. A risk stratification system that covers all rectal cancers and that incorporates the proximity of the primary rectal cancer to the mesorectal fascia, the depth of tumor invasion, the presence of metastatic lymph nodes, and the presence of venous invasion is used to classify LARC into “the good,” “the bad,” and “the ugly” [90, 91]. For their low-risk, “good” tumors, TME alone is recommended; for intermediate-risk “bad” tumors, the recommendation is short-course radiotherapy followed by TME; and for high-risk “ugly” tumors, the recommendation is CRT followed by TME. While this framework is intuitive, its utility has not yet been evaluated in prospective cohorts.
Selective Use of Chemotherapy
We can gain insight from studies that have sought to define the subgroups that benefit most from adjuvant therapy. Given the variable responses to CRT and its bearing on prognosis in LARC [2, 92], the need for adjuvant chemotherapy in patients who achieve a complete or near-complete response to CRT has been questioned [93]. A multi-institutional, retrospective analysis of 3133 patients recently revealed that the benefit of adjuvant therapy differs significantly between LARC subgroups. Patients with ypT1–2 or ypT3–4 tumors appeared to benefit more from adjuvant therapy compared with ypT0N0 patients, and those who achieved a pCR did not seem to benefit from adjuvant chemotherapy [94]. Some centers now use postoperative chemotherapy selectively, based on tumor response to CRT. In the recently published ADORE phase II trial, which examined the use of selective approaches to adjuvant chemotherapy, LARC patients with ypT3–4N0 or ypTanyN1–2 rectal cancers after CRT were randomized to receive adjuvant chemotherapy with either four cycles of 5-FU and LV or eight cycles of FOLFOX. The administration of FOLFOX after surgery was associated with prolonged progression-free survival (PFS) in stage III patients, but not in stage II patients [69]. In the coming years, more carefully conducted correlative studies will hopefully delineate the clinicopathologic characteristics and molecular markers associated with response to adjuvant therapy, which will prove useful in identifying which patients do not benefit from these treatments.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree