A thyroid nodule is defined as a discrete lesion in the thyroid gland that is radiologically distinct from the surrounding parenchyma.1 Thyroid nodules are very common in the general population, ranging from 20% to 76%. Nodules discovered at autopsy typically reflect this prevalence.2 Nodules are more common with increasing age, particularly in women.1 Up to 90% of women older than 70 years and 60% of men older than 80 years have thyroid nodules. Other risk factors include radiation exposure, particularly during childhood, family history of thyroid nodules, and iodine deficiency. Nodules may also occur in the setting of Hashimoto disease, where chronic inflammation leads to destruction of the thyroid gland with a rise in TSH in response to reduction in thyroid hormone. This rise in TSH stimulates growth of thyroid cells, forming nodules.
Thyroid nodules may be discovered by palpation during a physical exam in 3% to 7% of patients.3,4 Nodules may also be found incidentally on imaging studies such as neck ultrasounds, computed tomography (CT) scans, and deoxy-2[18F]fluoro-d-glucose positron emission tomography (18FDG-PET) scans performed for unrelated reasons. Risk of malignancy is the same in nodules discovered by palpation and those discovered incidentally.5
The proportion of thyroid nodules that prove to be malignant is 10% to 15%.6 Part of determination of malignancy is evaluation of individual risk assessment, which includes a detailed history and examination. Patients with the following histories may be at increased risk for malignancy:
1. Age. Patients age >70 years and <14 years have increased risk of malignancy.7 Nodules discovered during childhood have a three- to four-fold higher risk of malignancy than adults.8
2. Head and neck radiation, particularly during childhood. Nodules carry a 33% to 37% chance of malignancy.9
3. 18FDG-PET–positive lesion. See “Incidental 18FDG-PET Positive Lesions.”
4. Family history of thyroid cancer, particularly first-degree relatives.
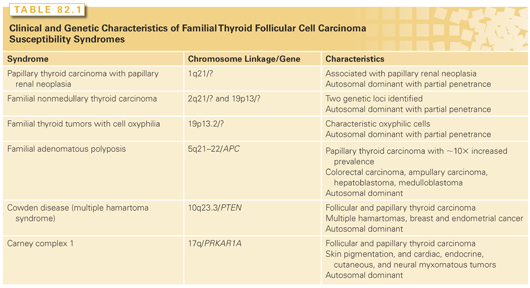
5. Personal history of genetic syndromes commonly associated with thyroid cancer (multiple endocrine neoplasia 2, familial papillary thyroid cancer, familial polyposis coli, Gardner syndrome, and Cowden disease; Table 82.1).
6. Physical examination findings of a fixed, hard, solid nodule, cervical lymphadenopathy, and vocal cord paralysis.
In addition to history and physical examination, a TSH level should be performed on discovery of a thyroid nodule. If the TSH is suppressed, then a radionuclear thyroid scan should be performed to ascertain whether the nodule is hyperfunctioning. Hyperfunctioning nodules are rarely malignant; therefore, further cytologic evaluation is not needed if the patient’s risk profile is otherwise low.7 It is generally accepted that if a cold nodule is present on radionuclear thyroid scan, cytologic assessment is performed. If the TSH is in the upper range of normal, there may be an increased risk of malignancy,10 although this association is poorly understood.
Routine measurement of calcitonin is controversial but is supported by the European Thyroid Association.11 The American Thyroid Association (ATA) cannot recommend for or against routine testing of calcitonin.1 It seems reasonable to perform, however, if there is a history of familial medullary thyroid cancer (MTC) as measurement may provide earlier diagnosis and more focused operative management.
A real-time two-dimensional, high-resolution ultrasound of the neck should be performed if thyroid nodules are discovered or suspected. Certain features may help distinguish benign from malignant lesions, although these signs are not independently predictive. These features include presence of microcalcifications, nodular hypoechogenicity, irregular borders, nodular vascularity, and a nodule that is taller than wide on transverse view.1 Purely cystic nodules are likely to be benign.12 Solitary nodules may not have a higher malignant risk than a nodule embedded in a multinodular goiter13,14; however, this still remains controversial.
Fine needle aspiration (FNA) is a very sensitive, low-risk procedure to help distinguish benign from malignant nodules. According to the Mayo Clinic experience from 1979 to 1990, FNA reduced the proportion of operations for benign nodules by 50% and increased detection of malignancy.15 The nondiagnostic rate is lower when performed in conjunction with neck ultrasound. Accuracy of the FNA will rely on an experienced team, and the cytopathologist should have an interest in thyroid disease. The ATA and American Association of Clinical Endocrinologists have provided guidelines to help clinicians decide which nodules require biopsy. Generally, routine FNA is recommended if the nodule is >1 cm with solid, hypoechoic features. Smaller nodules should also be considered for FNA if the nodule has suspicious features as described previously or if the patient has risk for thyroid cancer based on history or physical examination. The cytopathology may be reported as benign (60% to 80%), malignant (3.5% to 10%), suspicious for papillary thyroid cancer (2.5% to 10%), suspicious for follicular lesion/neoplasm or indeterminate (10% to 20%), and nondiagnostic (10% to 15%).7 Follicular or Hürthle cell adenomas and carcinomas appear identical on cytologic examination. Malignancy is found in approximately 20% of these neoplasms. Capsular or vascular invasion seen in the surgical specimen needs to be present for diagnosis of follicular or Hürthle cell carcinoma (HCC).
The use of genetic markers could help improve diagnostic accuracy of indeterminate lesions.16,17 Many centers now use these markers routinely to help guide management of indeterminate lesions, particularly in patients who do not want to undergo surgery. If the molecular profile suggests a low rate of malignancy (<5%), then observation may be recommended over surgical intervention.18 Long-term follow up studies are still needed before this technology is widely adopted.
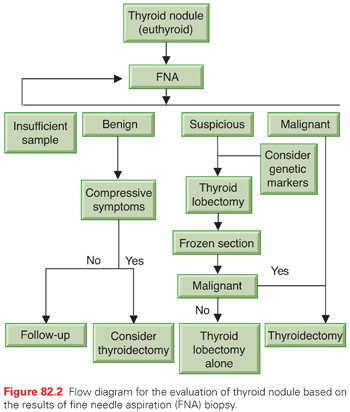
Figure 82.2 shows the evaluation of thyroid nodule in a euthyroid patient based on FNA results. Benign nodules can be followed conservatively by physical examination or ultrasound, unless there are compressive symptoms such as dysphagia or respiratory compromise, at which time surgical management should be considered. Lesions suspicious for follicular or Hürthle cell neoplasm typically are treated at Mayo Clinic with thyroid lobectomy and intraoperative frozen section. If the lesion is benign, no further thyroid resection is needed. If the lesion is malignant, completion thyroidectomy is performed. Molecular profiling can also be used to guide management decisions as described previously. Nondiagnostic specimens require repeat FNA, particularly if there are suspicious features on ultrasound. Repeated nondiagnostic FNAs may lead to surgical excision in order to obtain a diagnosis. Malignant lesions will be discussed in the chapter to follow.
Incidental 18FDG-PET Positive Lesions
Fluorodeoxyglucose whole-body positron emission tomography is frequently used in oncology to evaluate and follow-up malignancies. High concentrations of 18FDG occur in most malignant tumors; however, high glucose uptake may also be nonspecific. Normal thyroid tissue has diffuse, low accumulation of 18FDG. Incidental focal increased uptake in the thyroid may occur and has been reported in 1.1% of 18FDG-PET imaging in a recent series.19 Both primary and secondary thyroid malignancies have increased metabolic activity including lymphoma; therefore, an 18FDG-PET–positive solitary thyroid lesion has increased risk of malignancy of approximately 30% to 50%19–22 compared to the 5% risk of malignancy of an incidental thyroid nodule found by other imaging. Therefore, solitary 18FDG-PET–positive thyroid lesions should be referred for ultrasound-guided FNA to exclude a primary or metastatic lesion in the thyroid. Hot nodules have also been reported to have increased 18FDG uptake; therefore, if the TSH is suppressed, further imaging with a thyroid uptake and scan may be indicated. Some studies have advocated the use of maximum standardized uptake value to differentiate benign from malignant lesions20,21,23,24; however, this approach has not been supported by all studies.19,25 Diffuse thyroid uptake that does not correlate to a lesion on CT or ultrasound may be seen in autoimmune thyroid disease such as Hashimoto26 or Graves thyroiditis,27 and often do not require further evaluation unless other risk factors for malignancy are present.
Malignancies of the Thyroid
Risk Factors for Thyroid Malignancy
Although many risk factors have been evaluated in thyroid cancer, few clear associations have been found. Exposure to radiation, particularly in childhood, is associated with an increased risk of well-differentiated thyroid cancer.9 Relative risk is related to exposure dose, starting as low as 0.1 Gy.9 The latency period after childhood exposure is at least 3 to 5 years, and the risk remains apparent even 40 years after the radiation exposure.9 The majority of cases occur between 20 and 40 years after exposure. However, even after 40 years, the relative risk as compared to a nonirradiated population is still increased. In addition to those who are exposed to radiation for medical reasons, patients who have been exposed to nuclear disasters or who are atomic bomb survivors are also at increased risk for thyroid cancer. On the other hand, 131I for treatment of positive thyroid scans or hyperthyroidism has not been associated with this risk.
Family history is also an important risk factor, particularly if well-differentiated cancer is present in first-degree relatives28 or there is a family history of thyroid cancer syndromes as mentioned previously (see Table 82.1). MTC is also associated with distinct familial syndromes, which will be discussed in more detail later in the chapter.
The only known risk factor for thyroid lymphoma is Hashimoto’s thyroiditis,29 particularly the non-Hodgkin lymphoma derived from mucosa-associated lymphoid tissue.
THYROID TUMOR CLASSIFICATION AND STAGING SYSTEMS
The follicular cells give rise to well-differentiated cancers and anaplastic thyroid cancer (ATC). The C or parafollicular cell gives rise to medullary thyroid carcinoma. Immune cells and stromal cells of the thyroid are responsible for lymphoma and sarcoma, respectively. A total of 90% are well-differentiated cancers, 5% to 9% are medullary, 1% to 2% are anaplastic, 1% to 3% are lymphoma, and <1% are sarcomas or other rare tumors.30
Thyroid carcinoma can be categorized as tumors derived from follicular epithelium including differentiated thyroid carcinoma (DTC; papillary thyroid carcinoma, follicular carcinoma, including more aggressive forms such as HCC, some rare variants of papillary carcinoma, including the tall cell variant, columnar cell variant, and diffuse sclerosing variant), poorly differentiated thyroid carcinoma, and anaplastic carcinoma. Medullary thyroid carcinoma is derived from the C cells.
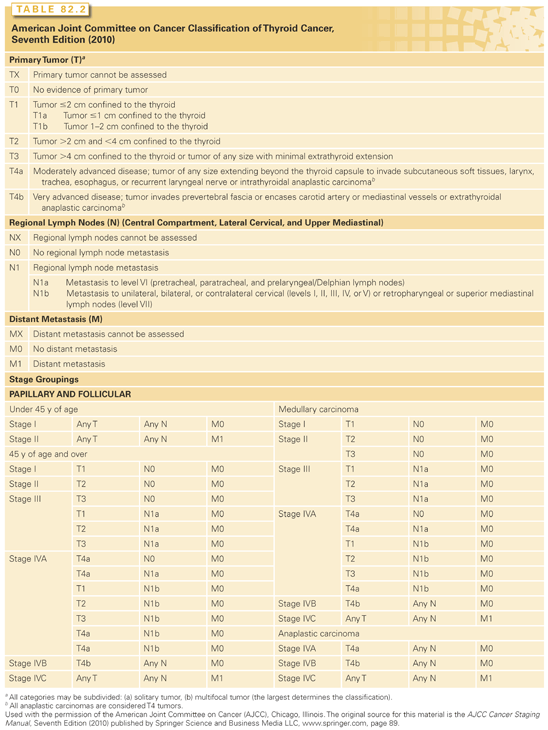
Many systems have been proposed for staging thyroid cancer. In the absence of a universally accepted system, it is recommended that the TNM (tumor-node-metastasis) staging system, introduced by the International Union Against Cancer and promoted by the American Joint Committee on Cancer, the American Cancer Society, the National Cooperative Cancer Network, and the American College of Surgeons, be adopted as the international staging system (Table 82.2). The system stages the malignant lesions based on tumor size, invasiveness, nodal spread, and distant metastases. Based on this system, all patients younger than 45 years of age with papillary or follicular carcinoma are stage I unless distant metastases are present. Patients older than 45 years of age with papillary or follicular microcarcinoma without nodal disease are stage I, whereas any tumor >2 cm is considered stage II. Stage III is classified in older patients with nodal disease or extrathyroidal invasion. Staging of MTC is similar, although there are no age distinctions and extrathyroidal invasion is classified as stage II. In papillary, follicular, and MTC, distant metastases are considered stage IV disease.
Incidence and Prognosis
In the United States, the diagnosis of thyroid cancer has tripled in the past three decades, likely secondary to increased detection rates. Papillary thyroid cancer (PTC) is by far the most common (80% to 85%) compared to 10% to 15% follicular and 3% to 5% HCC.31
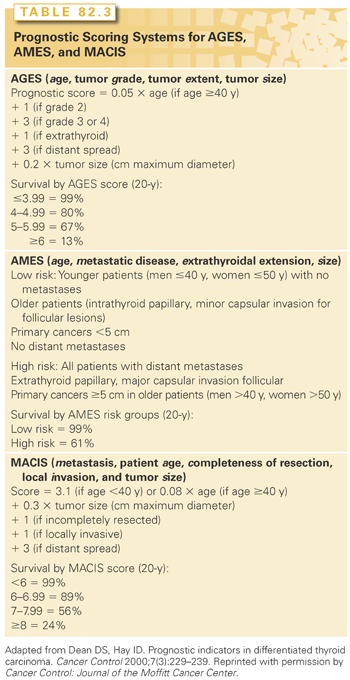
Several databases define prognostic risk factors for well-differentiated thyroid cancer.32 Most prognostic indicators are derived from uncontrolled retrospective trials. The most widely used systems include AGES (age, tumor grade, tumor extent, tumor size), AMES (age, metastatic disease, extrathyroidal extension, size), and MACIS (metastasis, patient age, completeness of resection, local invasion, and tumor size) (Table 82.3). Evaluation of 859 patients treated at Mayo clinic between 1946 and 1970 revealed that age of the patient, histologic grade of the tumor, and extent and size of the tumor were associated with worse prognosis.33 These variables were combined into a prognostic score (AGES) with high-risk patients having a score ≥4 and low risk patients <4. Cause-specific mortality in low-risk patients was 1.1% at 20 years compared to 39% in the high-risk group. The AMES system was developed after a subsequent analysis of 821 patients with DTC treated at Lahey Clinic between 1941 and 1980.34 This system was based on age, distant metastases, and extent and size of primary tumor. Twenty-year mortality rates were 1.2% and 39.5% for low- and high-risk patients, respectively. Worse prognostic indicators included male sex, older age, major capsular invasion for follicular carcinoma, tumors >5 cm in size, extrathyroidal extension of the tumor, and distant metastases at presentation. Mayo Clinic developed the MACIS system after analyzing 1,779 patients with PTC.35 A low-risk score is considered <6 with a 20-year mortality of 1%. This scoring system is the current predominant system to determine risk of death and postoperative outcome at Mayo Clinic, and is the only system to take gross residual disease after primary resection into account. These three staging systems allow the clinician to determine risk following the initial cancer operation.
Lymph node status has not been associated with cause-specific mortality; however, positive nodal involvement at presentation increases the risk of locoregional recurrence.36
A single point mutation (V600E) of the BRAF protooncogene is recognized as the initiating oncogenic trigger in up to 40% to 50% of PTC.37–39 Less commonly, RET rearrangements and RAS mutations may be present.40,41 When compared to papillary cancers that do not have BRAF mutations, PTCs with this mutation are more advanced at the time of diagnosis, more likely to be invasive beyond the thyroid capsule, more likely to metastasize to regional lymph nodes, more likely to exhibit distant metastatic spread, and therefore less likely to be surgically curable.37,42–46 It remains unclear whether BRAF V600E status provides incremental prognostic information beyond that available based on surgical and pathologic information obtained at the time of initial surgery. Nevertheless, some investigators suggest that assessment of V600E BRAF status could be a standard part of the workup for patients with PTC.47 For further discussion on the clinical and molecular genetics of endocrine tumorigenesis, see Chapter 81.
The majority (35% to 70%) of patients with follicular cell carcinoma and HCC present with stage II disease. In patients older than 45 years of age, stage III disease accounts for 4% to 7% of follicular thyroid carcinoma (FTC) and 8% to 10% of HCC. Stage IV disease with distant metastases is seen in 7% to 15% of FTC and 4% to 6% of HCC. Predictors of cause-specific mortality include age >50 years, marked vascular invasion, and metastatic disease at presentation.32 A retrospective review from Memorial Sloan-Kettering from 1930 to 198548 showed that age >45 years, Hürthle cell subtype, extrathyroidal extension, tumor >4 cm, and distant metastases were associated with worse prognosis. Patients were divided into low-, intermediate-, and high-risk groups. The 10-year survival for low-, intermediate-, and high-risk groups were 98%, 88%, and 76%, respectively. HCC is considered a more aggressive subtype, and studies evaluating flow cytometry show that tumors with DNA aneuploidy are associated with increased tumor-related mortality.49 Although originally designed for PTC, prognostic scoring systems AMES and AGES have been used in FTC, although it is important to point out that these scoring systems do not include important prognostic factors just described, namely vascular invasion or flow cytometry.
Pathology
PTC constitutes approximately 80% to 85% of malignant epithelial thyroid tumors. Grossly, papillary carcinomas have a variable appearance, from subcapsular white scars to large tumors >5 to 6 cm that invade nearby structures outside the thyroid gland. Cystic change, calcification, and even ossification may be identified. Microscopically, papillary carcinomas are characterized by the presence of papillae, but some variants contain no papillary areas, are totally follicular in pattern, and are identified as a follicular variant. Biologically, all these tumors, independent of their degree of follicular pattern, show similar clinical characteristics. The major cytologic feature shared by all members of this papillary group is the characteristic nucleus containing Orphan-Annie nuclei, nuclear grooves, and intranuclear pseudoinclusions. Because the nuclei are enlarged, they frequently overlap one another. Papillary carcinoma has a propensity to invade lymphatic spaces and, therefore, leads to microscopic multimodal lesions in the gland as well as a high incidence of regional lymph node metastases.
In contrast to the overall indolent behavior of the classical DTC, subtypes of these tumors have been identified as being more aggressive. These tumors comprise approximately 10% to 15% of all thyroid cancers.50 These include HCCs (oncocytic, oxyphilic) as well as variants of PTC such as the tall cell variant, columnar cell variant, and diffuse sclerosing variant. These variants exhibit unique histopathologic features. However, they do share some commonalities, such as a high rate of extrathyroidal extension and nodal metastasis at diagnosis, as well as locoregional recurrence and development of synchronous and metachronous metastasis.50
True FTC is a tumor comprising approximately 5% to 10% of thyroid malignancies in nonendemic goiter areas of the world.51 Most of the follicular pattern of thyroid malignancies represent the follicular variant of papillary carcinoma and share the biologic features, natural history, and prognosis of PTC.52 FTC is unifocal, thickly encapsulated, and shows invasion of the capsule and/or vessels.
The Hürthle cell neoplasm is considered by most to be a variant of follicular neoplasms. Historically, all such lesions, despite the histologic features, were considered to be malignant; hence, it was recommended that they all be treated aggressively. However, many studies have evaluated the clinical pathologic features of thyroid Hürthle cell tumors and have shown that, on average, only 20% to 33% show histologic evidence of malignancy or invasive growth and may metastasize.52 However, the size of the lesion is related to the risk of malignancy, and 65% of tumors >4 cm are found to be malignant.52 Hürthle cell tumors that do not demonstrate invasion microscopically behave as adenomas and may be treated conservatively.
TREATMENT OF DIFFERENTIATED THYROID CANCER
Surgery
Mayo Clinic has been observing the treatment of PTC since 1940.53 Over the decades, our management of PTC has become more aggressive. Seventy percent of patients treated for PTC in the 1940s underwent unilateral lobectomy. By the 1950s, the frequency dropped to 22% and by the 1960s, only 5% of patients with PTC underwent unilateral lobectomy. In contrast, by the 1980s, total or near-total thyroidectomy was performed in 92% of patients with PTC.53 In current day practice, a patient diagnosed with PTC will undergo either total or near-total thyroidectomy as the preferred initial treatment.
Preoperative ultrasonography of the neck allows for evaluation of suspicious neck node metastases (NNM) that should be resected with a modified neck dissection at time of initial thyroidectomy (Fig. 82.3). Of 1,916 patients undergoing primary surgery for PTC at Mayo Clinic during 1940 through 1991, 23% of patients had suspicious nodes by palpation. More than 50% of those with PTC had neck nodes removed at surgery; 38% of the patients with PTC had nodes that were positive for metastatic disease. Only 4% of patients with FTC and 6% of those with HCC had NNM at initial surgical diagnosis.54 Patients with DTC may be found to have recurrent NNM on long-term follow-up after initial surgery. The proportion of patients who present with these recurrences varies among histologic types of DTC. In 2,172 patients with DTC who underwent complete surgical resection at Mayo during 1940 to 1991, neck node recurrence occurred in 8% of PTC (1,801), 2% of FTC (124), and 18% of HCC (87) by 20 years. Definition of recurrence was 180 days after initial surgery.54 In 2,370 patients with PTC treated with curative intent during 1940 to 2000 at Mayo Clinic, the cumulative occurrence rates for postoperative NNM at both 25 and 40 years were 10%.55
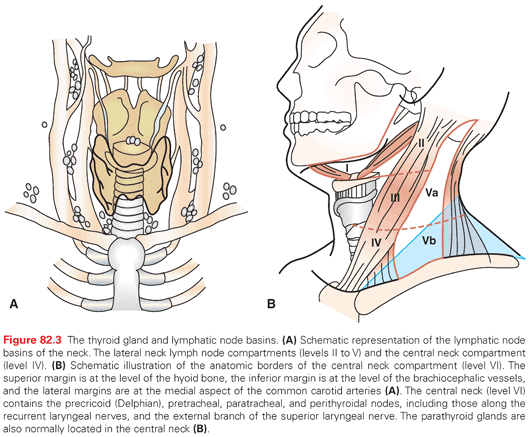
According to the European Thyroid Association,11 ATA,1 and National Comprehensive Cancer Network,18 therapeutic neck dissection is performed with initial thyroidectomy for patients with PTC with NNM. Prophylactic lateral neck dissection is not recommended; however, prophylactic central neck dissection remains controversial. At Mayo Clinic, we routinely dissect the central neck compartment at time of initial thyroidectomy considering the high risk of NNM in DTC. Contrast-enhanced CT scanning is indicated in patients with locally advanced disease or vocal cord paralysis to rule out invasion of the aerodigestive tree which will alter surgical management.
Adjuvant Therapy
Adjuvant therapy includes postoperative TSH suppression with thyroxine therapy, usually to a level around 0.1 mIU/L. If the patient is considered low risk, TSH levels are acceptable into the low range of normal. High-risk patients are often maintained at ≤0.1 mIU/L. This has been standard practice in PTC and FTC for many years; however, risks of thyroid hormone suppression should be weighed against individual patients’ cardiovascular risk.
The trends in surgical management of DTC have been described previously. Similar changes have been seen in the management of DTC with radioactive iodine (RAI), particularly with respect to postoperative radioiodine remnant ablation (RRA) in low-risk patients. At Mayo Clinic, approximately 60% of patients underwent RRA within 6 months of initial surgery in the 1980s. This is in comparison to only 6% in the 1970s.53 The ATA guidelines recommend RRA for patients with stage II disease older than 45 years of age and patients with stage III and IV disease. Only selected patients with stage I disease should be considered for RRA (aggressive histologies, multifocal disease, nodal metastases, extrathyroidal or vascular invasion).1 Analysis of 1,163 patients at Mayo Clinic with low-risk PTC (defined as MACIS <6) between 1970 and 2000 showed no statistical difference was seen in 20-year tumor recurrence and cause-specific mortality between those that received surgical treatment alone versus those who received surgical treatment with RRA.53 In addition, the National Thyroid Cancer Treatment Cooperative Study group56 and a multivariate analysis performed in Toronto, Canada,57 did not show improvement in recurrence and cause-specific mortality in patients with low-risk disease. It has therefore become clearer that RRA in low-risk disease does not change long-term outcomes and should be reserved for higher risk patients. At Mayo Clinic, we reserve postoperative RRA for patients with high-risk PTC (MACIS >6) or patients with a diagnosis of follicular or HCC. In those elected to receive RAI, quantitation of uptake is assessed by whole-body 131I scan and customization of dose is decided based on this quantitation and distribution of disease.
Postoperative RRA is typically performed approximately 6 weeks after near-total or total thyroidectomy. Most centers perform a pretherapy whole-body iodine scan. If performed, a pretherapy scan should use a low dose of 131I (1 to 3 mCi) or 123I. To optimize uptake by both normal residual thyroid and thyroid cancer, patients are rendered hypothyroid with a goal of increasing serum TSH. To accomplish this, thyroid replacement after thyroidectomy is often performed with the administration of triiodothyronine (T3), as it has a much shorter half-life than thyroxine (T4), and it is discontinued 2 weeks before treatment. In response to this hypothyroid state, TSH must be >30 mU/L to obtain optimal uptake of radioiodine. It is also recommended that a serum thyroglobulin level is obtained during this period of hypothyroid state (see “Long-Term Surveillance”). A low-iodine diet is recommended 1 to 2 weeks before scanning or ablative 131I therapy to enhance the uptake and retention of radioiodine. Alternatively, imaging and treatment employing TSH stimulation with recombinant human TSH (rhTSH; Thyrogen, Genzyme, Cambridge, MA) is presently performed with increased frequency. This way is preferred if rendering the patient frankly hypothyroid is potentially hazardous, for example, metastatic PTC in the central nervous system. Posttherapy whole-body iodine scanning is typically performed 1 week after 131I treatment to identify metastases.
The most common side effects from radioiodine therapy include sialadenitis, nausea, and temporary bone marrow suppression. Women undergoing 131I treatment should be advised to avoid pregnancy during and 6 to 12 months after treatment due to risk of miscarriage and fetal malformation. There is a weak, but dose-dependent relationship between 131I therapy and the development of second malignancies, such as bone and soft tissue tumors, colorectal cancer, salivary tumors, and leukemia.58
Papillary Thyroid Microcarcinoma
Papillary thyroid microcarcinomas (PMC) are defined as PTC with a maximum diameter of ≤10 mm.59 In contrast with ATC with a poor prognosis, PTC generally has an excellent prognosis, with majority of patients with small primary tumors alive at 20 years. Prior to the advent of high-resolution ultrasound, most PTCs were discovered by palpation. With improved ultrasound imaging techniques and the ability to perform guided biopsies in the office setting, subclinical papillary cancers are now being discovered. In addition, these micropapillary carcinomas are often incidentally found on imaging performed for other reasons. Autopsy studies reveal an incidence of 6% to 36%.60 The treatment of these microcarcinomas remains very controversial in the thyroid field. Mayo Clinic reported treatment outcomes of 900 patients with PMC over 60 years.61 Prognosis was excellent with cause-specific mortality of only 0.3%. A total of 30% of patients had cervical lymph node involvement, with only 0.3% having distant spread at the time of diagnosis. Bilateral lobar resections were performed in 86% with unilateral lobectomy performed in 14%. By 20 years, 5.7% of patients recurred within the neck or at distant sites. Eighty percent of recurrences occurred within cervical lymph nodes, whereas the remainder occurred in the nonresected contralateral lobe or soft tissues of the thyroid bed. However, cervical recurrences were not associated with increased mortality. Of the recurrences, 4% were to distant sites. Higher recurrences were seen in patients with multifocal tumors and positive NNM at primary resection. Recurrence rates did not differ whether primary resection included bilateral or unilateral lobar resection. RRA did not improve mortality or recurrence rate. In summary of this large series, as long as complete resection is possible, more extensive surgery and RRA do not seem to influence recurrence risk or mortality. The most common site of recurrent disease is the cervical lymph nodes, and central compartment exploration can be performed at the time of initial surgery to remove involved nodes. Future recurrence is higher in those with positive nodes at initial resection, and those with multifocal disease.
In a Japanese observational study of 1,395 patients with PMC, patients were given the choice of surgery versus observation if no unfavorable features were present.62 Less than a quarter chose observation, and these patients were followed with frequent ultrasounds over an average of 74 months. Tumor enlargement was considered growth of ≥3. At 5- and 10-year follow-up, the proportion of patients with tumor growth was 6.4% and 15.9%, respectively. Eighteen of the thirty-one patients with tumor enlargement underwent surgery after observation, two of which were found to have lymph node metastases. Of the 13 patients who continued with observation, 7 tumors decreased in size. Tumor enlargement was not related to the original size of the tumor, multicentricity, or TSH suppression. Tumors of patients under the age of 45 tended to enlarge, but this did not reach statistical significance. The proportion of patients with new node metastases at 5- and 10-year follow-up was 1.4% and 3.4%, respectively. This author therefore summarized that observation of papillary microcarcinoma without unfavorable characteristics is a potential therapeutic option, with progression to surgical resection if tumor enlargement or lymph node metastases occur.
Treatment of PMC is largely based on observational and epidemiologic studies. Further large trials are needed to rest this controversy. Recently, authors have advocated for a change in the name of PMC to try and prevent overtreatment of these small tumors.63 However, it is important to note that not all PMCs may be “created equal,” with more aggressive variants of PTC possibly behaving differently.64 It is currently unknown whether these aggressive variants change survival risk.
Long-Term Surveillance
The goal of long-term surveillance is to identify recurrence in patients thought to be free of disease. Thyroglobulin (Tg), an important serum tumor marker in the surveillance of patients with thyroid cancer, is the protein that provides a matrix for thyroid hormone synthesis within thyroid follicles and is critical in the storage of thyroid hormone within the thyroid gland. After successful thyroidectomy and ablation of residual normal or malignant thyroid tissue by radioiodine, the Tg should be in the athyreotic range. Both normal thyroid tissue as well as follicular cell–derived thyroid cancer will produce Tg. Levels above the athyreotic range are indicative of persistent, functioning thyroid tissue or carcinoma.
Follow-up protocols have also been a controversial issue in the management of PTC. At Mayo Clinic, we typically order a TSH with Tg testing (including Tg tumor marker and antibody) yearly for at least 5 years as this is the period of time where most recurrences occur. The presence of autoantibodies to Tg, which occurs in 25% of patients with thyroid cancer and 10% of the general population, will falsely lower serum Tg levels. Thus, such antibodies should quantitatively be determined at every measurement of serum Tg levels. We do not routinely order a TSH-stimulated Tg level. In patients with low-risk disease, a TSH-suppressed Tg level <0.5 ng/mL likely represents absence of clinically relevant disease.
In addition to Tg and TSH monitoring, follow-up imaging after initial surgery and adjuvant therapy is required. High-sensitivity ultrasonography has become the mainstay of imaging surveillance in patients with low-risk disease, with the ability to pick up small NNM that are millimeters in size. Neck ultrasound should be performed 6 and 12 months after surgery, and then annually for 3 to 5 years, depending on the patient’s risk for recurrence and Tg status.1 At Mayo Clinic, routine posttherapeutic radioiodine whole-body scanning (WBS) in the absence of recurrence by ultrasound or positive Tg is not performed. A report from Mayo Clinic Jacksonville looked at 194 patients with follicular cell–derived thyroid cancer with a TSH suppressed Tg of 0.1 ng/mL who subsequently underwent TSH stimulation and radioiodine scanning.65 The Tg rarely stimulated above 2 ng/mL and none of the 194 patients had radioiodine scanning that was positive for locoregional recurrence or distant metastases. Therefore, radioiodine scanning is reserved for patients with recurrence or metastatic disease that may be amenable to therapeutic doses of RAI.
Management of Local Recurrence and Distant Metastasis
Patients with significant nodal locoregional recurrence in the neck should undergo modified radical neck dissection or central compartment (level VI) neck dissection, depending on the location of the recurrence. More aggressive surgery may be warranted in selected patients with invasion of the aerodigestive tract.66 Tracheal stents and tracheotomy can be used as palliative measures in the case of unresectable disease or poor performance status. For smaller regional lymph node metastasis or patients who are not amenable to surgical therapy or have distant metastasis, locoregional disease control can also be achieved using ultrasound-guided percutaneous ethanol ablation, which will be discussed in more detail in the following.
Metastatic disease that is detected with whole-body iodine scan, and is considered radioiodine avid, is treated with 131I therapy. Similar to the discussion of initial treatment, no consensus exists regarding dosing of 131I, although most authors use a high dose ranging between 150 to 300 mCi. Pulmonary metastases are frequently detected exclusively on radioiodine scanning and tend to respond to 131I treatment. Treatment can be performed every 6 to 12 months as long as the disease continues to respond. It should be noted, however, that pulmonary fibrosis may limit further 131I treatment.67 For select patients with incurable pulmonary disease, palliative treatments using metastasectomy, laser ablation, and external beam radiation therapy (EBRT) may be considered. Complete surgical resection of isolated symptomatic bone metastases and131I treatment for radioiodine avid, widespread disease have both been associated with an increased survival and are recommended especially in younger patients.67
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








