PATIENT SELECTION USING QUANTITATIVE PROGNOSTIC INDICATORS
A second factor strongly recommending CRS and HIPEC as a valid treatment option comes from well-established selection factors applied to this patient population. With some variations between diseases, the same group of quantitative prognostic indicators operates for all patients with peritoneal surface malignancy. Now, prognostic indicators are used to refine the selection of patients to those most likely to benefit and to exclude those who are unlikely to benefit.15,16 Histopathology, the peritoneal cancer index (PCI), the completeness of cytoreduction score (CC), radiologic imaging by computed tomography (CT), and TNM (tumor, node, metastasis) stage with peritoneal cytology play a central role in patient selection.
Biologic Aggressiveness as Measured by Histopathology for Epithelial Appendiceal Neoplasms and Peritoneal Mesothelioma
Mucinous appendiceal neoplasms have a broad spectrum of aggressiveness, and the histologic type of the appendiceal neoplasm has a profound effect on survival following treatment by CRS and HIPEC.17 Patients with adenomucinosis obtain maximal survival benefit, while those with mucinous carcinoma show survivals similar to that for peritoneal metastases of colorectal origin.18
Seven different histologic patterns of peritoneal mesothelioma contribute to three distinct histologic groups that have a very different prognosis following treatment with CRS and HIPEC: poor prognosis for sarcomatoid, deciduoid, or biphasic types; intermediate prognosis for papillary and epithelial types; and excellent prognosis for low-grade or multicystic types.19,20
Extent of Disease as Measured by the Peritoneal Cancer Index
The PCI is an assessment combining lesion size (0 to 3) with tumor distribution (abdominopelvic regions 0 to 12) to estimate the extent of disease within the abdomen and pelvis as a numerical score (Fig. 115.2).21 It is calculated from observations obtained at the time of surgical exploration of the abdomen and pelvis, and should be recorded by the surgeon before leaving the operating room. The higher the PCI, the less likely CRS and HIPEC will result in long-term survival for all the peritoneal surface malignancies discussed in this chapter.
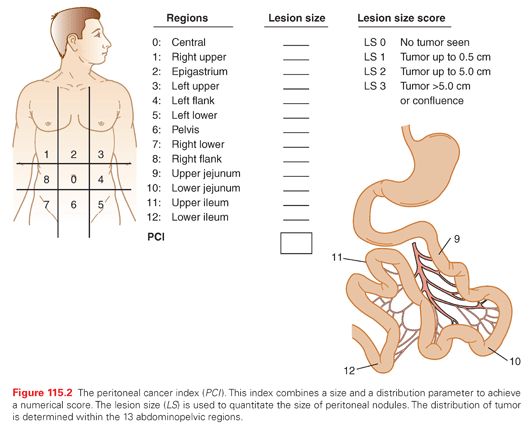
The Completeness of Cytoreduction Score Performed after the Resection
The size of residual tumor nodules visually determined after CRS has been completed has a profound effect on outcome. The new definition of complete cytoreduction is no visible evidence of cancer or only minute nodules reliably penetrated by HIPEC, a completeness of cytoreduction score of 0 (CC-0) or CC-1, respectively. With few exceptions, a CC-0 or CC-1 score is necessary for long-term benefit with CRS and HIPEC.21
Preoperative Radiologic Imaging by Computed Tomography
CT of the chest, abdomen, and pelvis is an essential tool in the selection of patients for CRS with HIPEC. Systemic metastases and spread to pleural surfaces can be identified. The location and quantity of mucinous carcinoma within the peritoneal cavity can be accurately determined.22,23 Unfortunately, nonmucinous peritoneal metastases may be greatly underestimated by CT.24 If the small bowel and its mesentery are layered by tumor or a large mass of cancer occupies the epigastric region, the likelihood of achieving complete cytoreduction is small. The CT should be performed with maximal intravenous and oral contrast in order to identify patients who have small bowel compartmentalization versus diffuse involvement of the small bowel and its mesentery.
Tumor, Node, Metastasis Stage, Peritoneal Cytology, and Clinical Features of the Primary Cancer
A careful survey of the peritoneal surfaces at the time of primary gastrointestinal cancer resection combined with a knowledgeable and timely gross and histopathologic assessment of the resected specimen can accurately predict the incidence of peritoneal metastases that will occur in follow-up. Also, in advanced gastrointestinal cancers, peritoneal cytology provides important prognostic information. This information should be used by the MDT to consider proactive treatment with CRS and HIPEC. It is possible to prevent peritoneal metastases in high-risk groups or treat peritoneal metastases by second-look surgery in patients with documented disease.25–30 The fast pace at which peritoneal metastases progress and the profound effect of extent of disease (PCI) on outcome indicate this approach. Table 115.1 presents the clinical features that should alert the MDT to a high risk for peritoneal metastases.31,32
Pharmacokinetic Advantage of Perioperative Chemotherapy
Another argument in favor of CRS and HIPEC as a treatment option for peritoneal surface malignancy is a well-studied pharmacologic rationale. As determined by physical properties, some chemotherapy agents are especially appropriate for hyperthermic use in the peritoneal cavity after CRS has been completed. Other cell cycle–specific chemotherapy agents are more appropriate for EPIC. As experience with different perioperative chemotherapy regimens has accumulated, the use of multiple-agent chemotherapy given both intravenously and intraperitoneally has evolved and promises to be increasingly effective. Heat targeting of both intravenous and intraperitoneal chemotherapy to the peritoneal surface cancer nodule is a goal of treatment.
Combined Use of Cytoreductive Surgery and Perioperative Chemotherapy as a Standard of Care in Selected Patients
A final argument for use of CRS and HIPEC as a treatment option for peritoneal surface malignancy concerns the well-defined management parameters that are currently in practice at experienced treatment centers. A standardized cytoreductive surgical procedure involves visceral resections and peritonectomy procedures in an attempt to leave the abdomen visibly free of cancer. After the CRS is complete, tubes and drains are positioned to facilitate inflow and outflow of the chemotherapy solution. The chemotherapy solution circulates through roller pumps and a heat exchanger to maintain moderate hyperthermia (42°C/109°F) within the abdomen and pelvis. The skin edges are elevated on a self-retaining retractor (open method) or the skin closed (closed method) in order to create a reservoir for the hyperthermic chemotherapy solution.
The sequence of cancer resection, intraoperative chemotherapy washing, and then intestinal reconstruction may be important to optimize the destruction of small volumes of cancer cells and prevent tumor cell entrapment in adhesions, suture lines, and the abdominal incision. During the peritonectomy and visceral resections, large volumes of cancer are removed; however, residual cancer cells remain and must be eradicated.
A review of the important recent contributions to the literature that critically assesses the benefits of CRS and HIPEC in different disease states constitutes the remainder of this chapter.
Natural History
Although similarities exist, there are unique features of appendiceal malignancies as compared to colorectal adenocarcinoma. The first and probably the most obvious difference between the colon and the appendix malignancies is the diameter of the bowel lumen involved. A colon tumor grows as an intraluminal mass in the bowel and will invade through the wall. The intraluminal growth pattern causes the primary cancer to reach the serosa in a late stage. In contrast, the appendix is a small organ, up to 1 cm in diameter, with a thin wall. A tumor may obliterate the appendix lumen in an early stage. This may cause a rupture of the wall of the appendix, allowing a spreading of tumor cells into the abdominal and pelvic cavity. Early in the natural history of the disease, these neoplastic cells implant with a high efficiency throughout the peritoneal cavity and progress as peritoneal metastases.33,34
Histologic Classification
The biology of appendiceal malignancies varies from simple mucinous adenoma to solid intestinal type adenocarcinoma. The histology of the peritoneal metastases from the primary tumor itself has a major influence on the outcome of the treatment by CRS and HIPEC. Ronnett et al.35 and Bruin et al.36 described three different types in their analyses of appendiceal malignancies. Metastases with >90% mucus, flat epithelial cells, no atypia, and no mitoses had a good prognosis despite a high PCI. At the other end of the spectrum, peritoneal metastases with much atypia, abundant mitoses, and <50% mucus has a prognosis similar to colorectal carcinomatosis and is referred to as peritoneal carcinoma.
Outcome of Treatment of Peritoneal Dissemination by Cytoreductive Surgery Followed by Hyperthermic Perioperative Chemotherapy
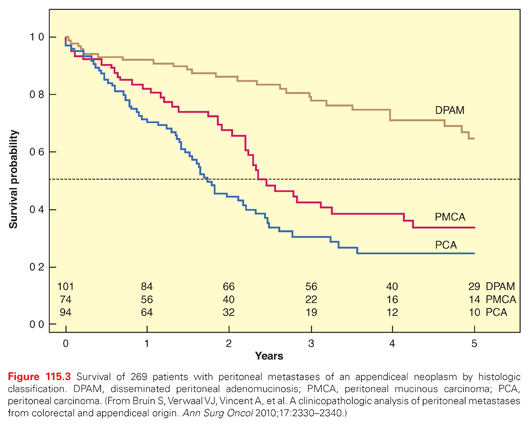
The survival outcome with peritoneal dissemination of appendiceal origin neoplasms is shown in Figure 115.3. The overall survival is shown according to the histologic classification. For the least aggressive form (disseminated peritoneal adenomucinosis), the median survival is not reached within 5 years.36 Those patients who were affected by peritoneal carcinoma had a much poorer prognosis. The median survival was only 14 months in these patients. These survival data compare with the median survival of peritoneal metastases of colorectal origin treated with cytoreduction followed by HIPEC. Accepting the fact that complete resection is always the goal of CRS, these data document that survival with peritoneal metastases from appendiceal malignancies is highly dependent upon the histopathologic characteristics of the tumor.
Success in the treatment of recurrence after primary treatment with CRS followed by HIPEC is a unique feature of appendiceal peritoneal metastases. Benefit depends largely on the number and location of tumor masses. With a limited number of locations, the first choice is resection; if more diffuse disease is present and CRS is complete, a second HIPEC can be performed.37,38 In patients in whom the pathology is more aggressive, definitive treatment with systemic chemotherapy, with all it limitations, should be considered by the MDT.
Surgical Learning Curve
Cytoreduction followed by HIPEC is a complex treatment that demonstrates a surgical learning curve.39,40 The peak of the learning curve in the study of Smeenk et al.31 was reached after approximately 130 procedures. Surgical skill is undoubtedly the main component of this learning process, but modified treatment strategies for an individual patient and experience in handling complications by the entire medical team has contributed to a decreased morbidity and mortality.41,42
COLORECTAL PERITONEAL METASTASES: CURATIVE TREATMENT AND PREVENTION
Stage IV colorectal cancer is a very morbid disease, with a 5-year survival rate of 10% and a median survival of 14.4 months. The prognosis is significantly worse when peritoneal metastases are present, with a median survival of 6.7 months versus 18.1 months without peritoneal metastases (p <0.01).43 When colorectal peritoneal metastases are diagnosed, the crucial question for the MDT is whether curative (complete CRS with HIPEC) or palliative therapy (systemic chemotherapy) should be the goal of treatment.
Incidence of the Disease and Incidence of Peritoneal Metastases
At the initial diagnosis of colon cancer, the peritoneal surface is involved with tumor in 10% to 15% of patients.11,44 Besides the liver, the peritoneal surfaces are the most common sites of cancer recurrence after “curative” colorectal cancer resections. Unfortunately, cancer progression occurs in as many as 50% of patients who have an R0 resection. In 10% to 35% of patients with recurrent disease, the anatomic site of treatment failure is confined to the peritoneal surface.11,44 With recurrent disease, when peritoneal metastases are of limited extent (low PCI), potentially curative treatment options should be considered.
Useful Prognostic Indicators for the Disease
Contraindications for this combined treatment are the presence of metastases at another site (although, up to three easily resectable liver metastases is not an absolute contraindication),45 a poor general status (the combined treatment is rather aggressive), and extensive peritoneal metastases (PCI >20). Extensive disease is likely to be unresectable in its entirety. Also, even when a complete cytoreduction is possible, the prognosis is poor. Most of the series using complete CRS with HIPEC report a poor survival rate when the PCI is higher than 20.46,47
Results of Treatment
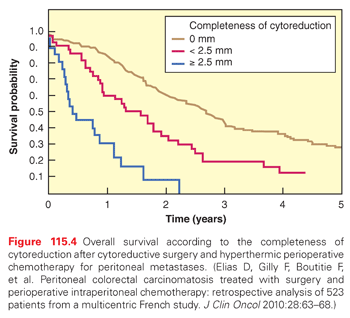
Figure 115.4 reports the survival rates according to the completeness of surgery in the 523 patients collected by the Association Française de Chirurgie.47 Five years after surgery, no patient was alive if the size of the residual tumor nodules exceeded 2.5 mm after surgery, whereas 30% of the patients were alive if complete CRS was possible (p <0.0001). These data are confirmed in the Dutch HIPEC study, which included 960 patients.48
A crucial role for hyperthermia for HIPEC is currently unknown for humans. In a randomized trial in rats, those treated with HIPEC survived longer than those treated with intraperitoneal chemotherapy alone or with intraperitoneal hyperthermia alone.49 A recent retrospective study compared similar patients (all of whom underwent a laparotomy) with resectable peritoneal metastases treated with complete CRS and HIPEC to patients who received standard systemic chemotherapy. Median survival was 63 months in the complete CRS with HIPEC group versus 24 months in the systemic chemotherapy group.50
Summary of Important Results from Other Institutions
This combined treatment (complete CRS and HIPEC) is effective. Benefits were definitively demonstrated by the randomized study of the Amsterdam group: 105 patients who presented with colorectal peritoneal metastases were randomized between surgery with HIPEC and systemic chemotherapy versus systemic chemotherapy alone.51 In this study, close to 50% of the patients in the experimental arm were at presentation not good candidates for HIPEC because their peritoneal metastases could not be completely resected. Despite this bias, the reported median survival was 22 months in the experimental arm versus 13 months in the control group (p = 0.032). At eight years the benefit persisted.52
The results obtained with complete CRS with HIPEC in the Association Française de Chirurgie study showed a 5-year overall survival of 30%.47 This result accepts clinical data over an 18-year interval and includes the learning curves of 28 different centers. It is the least favorable data ever to be reported by the authors’ group. The results of experienced centers concerning patients who underwent complete CRS and HIPEC show 5-year overall survival rates close to 40%. It was 32% for the 70 patients in the study by da Silva et al.,46 43% for the 59 patients in the Verwaal et al.51 study, and 48% for the 30 patients treated by Elias et al.53 Such a survival rate has never been published before for colorectal peritoneal metastases, even if the report includes only selected patients.
Furthermore, these results in the treatment of peritoneal metastases are similar to those obtained with hepatectomy for liver metastases. Selected patients with peritoneal metastases should be treated with this combined therapy just as selected patients with liver metastases should be treated with hepatectomy given that similar results are achieved in terms of survival.54
Prevention of Peritoneal Metastases in Patients at High Risk for Progression
Unfortunately, early diagnosis of peritoneal metastases is not possible with radiologic imaging, but only with a laparotomy. This is why the authors proposed second-look surgery plus HIPEC in asymptomatic patients who presented with a primary exhibiting a high risk of developing peritoneal metastases.28,55 High-risk patients were those who presented with a few (resected) peritoneal implants, ovarian metastases, or perforated tumors. Second-look surgery was performed 6 months after the end of the classic 6 months of systemic adjuvant chemotherapy. Among 47 patients, macroscopic peritoneal metastases was found and treated in 50% of them. In the remaining patients with no macroscopic peritoneal metastases, peritoneal recurrence occurred frequently in those who did not receive HIPEC, but rarely in those who did receive HIPEC (p = 0.02). Finally, only 17% of the patients treated at the time of second-look surgery with HIPEC developed a peritoneal recurrence.28
Reports of complete CRS and HIPEC being used to simultaneously treat the primary colorectal cancer and the peritoneal metastases have appeared.25–27 Also, patients without established peritoneal disease but at high risk for subsequent peritoneal progression are being treated with HIPEC during the same procedure used to resect the primary tumor.26 The rationale for this proactive approach is three-fold: First, after the primary cancer resection and complete CRS, the PCI was maximally low. Second, reliable radiologic tests to detect the progression of a low volume of peritoneal metastases do not exist. And third, this proactive approach does not require a second-look surgery. With rectal cancer, a second-look approach with CRS and HIPEC may rarely, if ever, be curative.46,56
DIFFUSE MALIGNANT PERITONEAL MESOTHELIOMA
Clinical Presentation and Diagnosis
Diffuse malignant peritoneal mesothelioma (DMPM) is characterized by progressive peritoneal seeding, eventually leading to the patient’s death due to tumor layering on peritoneal surfaces, bowel obstruction, and intractable malignant ascites. Patients are usually diagnosed with presenting signs and symptoms of advanced disease. In a recently published series of 81 patients with DMPM reported by an Italian group, ascites, abdominal pain, and asthenia were the most frequent symptoms present on 77%, 69%, and 43% of patients, respectively. CT scan shows ascites, peritoneal thickening, abdominal mass, and mesenteric thickening, respectively, on 80%, 63%, 32%, and 29% of cases.57
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree