The stem cell and to some extent the committed progenitors have high self-renewal potential and low proliferative rate, while the differentiated marrow precursors have a low self-replicative capacity and high proliferation. Low proliferation protects the stem cells and the committed progenitors from cytotoxic chemotherapy that is cycle active and damages preferentially proliferating cells. In addition, the earliest progenitors express the MDR-1 gene that encodes the P-glycoprotein, a calcium channel pump extruding natural genotoxic products from the cell.12 These include both tumor antibiotics and plant derivatives. Thanks to the preservation of the hematopoietic reserve, the myelodepression of chemotherapy is generally fully reversible. There are exceptions, however. Some alkylating agents including busulfan, melphalan, nitrogen mustards, and the nitrosureas, and the antibiotic mitomycin C are able to overcome the protective mechanisms and to destroy the hematopoietic progenitors.13,14
Hematopoiesis requires an intact hematopoietic stroma capable to home the early progenitors, and a network of growth factors that stimulate proliferation, commitment, and differentiation11,15–18 (Table 133.1). The homing occurs in the so-called hematopoietic niches that bind the hematopoietic progenitors through specific receptors to endothelial cells and osteoblasts. Except for erythropoietin that is produced in the kidney, and thrombopoietin that is produced both in the liver and in the kidneys, the other hematopoietic growth factors are produced by monocytes and by stromal cells of the bone marrow.

Hematopoiesis may be altered due to a reduction in the number of progenitors, the development of abnormal progenitors that have lost their ability to self-replicate or commit, abnormal production of hematopoietic growth factors, and abnormalities in the hematopoietic microenvironment. Some of these changes do occur with aging and include loss of homing, increased concentration of stem cells with reduced self-renewing capacity, and preferential differentiation of the totipotent stem cell toward the myeloid rather than the lymphoid series.19,20
Neutropenia and Its Management
Definition of Neutropenia and Its Consequences
According to the Common Terminology Criteria for Adverse Event version 4.0, neutropenia is defined by a granulocyte count that is ≤1,500/μl,21 and neutropenic infection is assumed for any fever episodes occurring when the neutrophil count is <1,000/μl. When the neutrophil count is <500/μl, the risk and severity of infection is inversely related to the neutrophil count.22 The duration of neutropenia also determines the type of infections.2 Bacterial infections from gram-negative pathogens and Staphylococcus aureus are more common during the first week of neutropenia.23 A number of fungal infections including Candida, Aspergillus, Mucor, and others may develop in patients with more prolonged neutropenia including those receiving treatment for acute leukemia.24 In addition to the severity, the duration of neutropenia determines the risk of infection. Infections are rare during the first 3 days of neutropenia as the neutrophils may still be present in the tissues.
For management purposes, it is useful to identify two different conditions: the neutropenia that follows marrow ablation for the treatment of some forms of acute leukemia, and the neutropenia that develops during the treatment of solid tumors or lymphomas with cyclic cytotoxic chemotherapy. Following marrow ablation, the neutropenia may last several weeks and the risk of infections is >90%.22 The main concern in this situation is support of the patient until marrow regeneration.
The neutropenia following noncytotoxic chemotherapy of solid tumors and lymphomas causes two concerns: infectious complication and maintenance of treatment dose intensity. This may be compromised due to treatment delay or dose reductions in order to prevent neutropenia. The relative dose intensity (RDI) is the percentage of the planned treatment dose administered during a course of treatment per unit of time and is calculated25 as:

where D is the dose and T the time unit.
The effectiveness of chemotherapy may be determined by the RDI, whose preservation is essential in curable diseases. When the dose intensity of adjuvant cyclophosphamide, methotrexate, and fluorouracil was <85%, the treatment was not effective in women with early breast cancer.25 Likewise, a reduction of the dose density of doxorubicin <85% was associated with reduced response rate and survival in patients with B-cell large-cell lymphoma treated with cyclophosphamide, hydroxydaunorubicin, oncovin, and prednisone (CHOP).25 Neutropenia has been responsible for at least 50% of the cases in which the chemotherapy dose intensity has been reduced below 85%.25
The neutrophil kinetics determines the course of chemotherapy-induced neutropenia.26 The median survival of mature neutrophils is approximately 12 hours in the circulation and 3 days in the tissues. The bone marrow contains a reserve of mature granulocytes to last approximately 7 days. The development of mature granulocytes from committed myeloid progenitors takes approximately 10 to 14 days. Consequently, the nadir neutrophil count following chemotherapy every 3 or 4 weeks is expected around the 10th treatment day, with recovery beginning around the 14th and completed by the 21st day. The time-honored way to prevent neutropenia and neutropenic infections prior to the introduction of myelopoietic growth factors included reduction of the doses of myelotoxic agents for a neutrophil count <500/μl at nadir or <1,500/μl at the beginning of the new treatment cycle, whose initiation was delayed until the neutrophil count was completely recovered.
The neutrophil kinetics may be altered by a number of factors. Drugs that affect the pluripotent progenitors, such as mitomycin C and nitrosureas,27 may cause later, more prolonged and cumulative neutropenia. In the presence of infection, the neutrophil reserve of the bone marrow may be exhausted at an earlier time and neutropenia may be more prolonged due to increased utilization of mature neutrophils. Also, hematopoiesis may be compromised by a number of factors that include age, previous chemotherapy administration, and myelophthisis.
Risk Factors for Chemotherapy-Induced Neutropenia
Clearly, the risk of neutropenia varies from patient to patient and includes both treatment- and patient-related factors. The determination of the individual risk of neutropenia and neutropenic infections is helpful to plan the most effective management of neutropenia. A number of risk factors for febrile neutropenia were identified in several studies28–30 (Table 133.2).
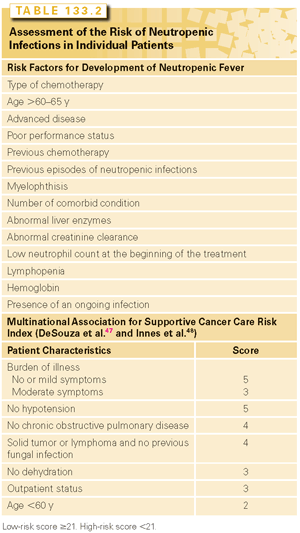
The risk of neutropenic infections from different treatment regimens is obtained from the original trials of those regimens. It should be underlined that the incidence of neutropenic infections may be higher in patients treated in the community than in those included in clinical trials.28–30
Age is probably the best identified patient-related risk factor. It is clear that the risk of neutropenic infections increases with age. A review of 500 patients with large-cell B-cell lymphoma treated in the community with CHOP or cyclophosphamide, novantrone, oncovin, and prednisone showed that the risk of neutropenic infections was 28% for the total population, 18% in the younger individuals and 40% for those 65 and older.5 Hospitalization for neutropenic infections was 25% longer for older individuals. This finding is important as both treatment cost and risk of deconditioning increase with hospitalization.
In most studies the age threshold was 65, and in some it was 60. The risk of breakthrough infection despite growth factor support30 and of infectious mortality also increases with age.3,4
In the Surveillance, Epidemiology and End Result database, the risk of febrile neutropenia in Medicare recipients increased with the number of comorbid conditions.31 In other studies, chronic liver and kidney dysfunctions, chronic obstructive lung disease, diabetes, congestive heart failure, osteoarthritis, and thyroid diseases were associated with increased risk of febrile neutropenia.10,32
In several studies, anemia was an independent risk factor for the development of chemotherapy-induced neutropenia and neutropenic fever.33–38 Anemia may represent a sign of chronic diseases, of bone marrow insufficiency, and of malnutrition.39 Another explanation is suggested by pharmacokinetic considerations.34 As the majority of cytotoxic agents are bound to the red blood cells, a reduction of hemoglobin may be associated with increased concentration of free drug in the circulation and consequently with increased risk of toxicity.
Protein calorie malnutrition also appears as a risk factor for chemotherapy-induced myelotoxicity.40–43
Myelophthisis may occur with most tumors and is particularly common in patients with lymphoma, breast, and prostate cancer.
The relationship between lymphopenia and risk of neutropenic infections is well established albeit poorly explained.44–46 A drop in lymphocyte count during the first week after chemotherapy seems predictive of febrile neutropenia. A possible explanation is that lymphopenia represents poor nutrition.
A number of models to predict the risk of neutropenia or neutropenic fever in solid tumors and lymphomas were developed. The five models that have been prospectively validated are described here. The Multinational Association for Supportive Cancer Care risk index illustrated in Table 133.2 divides the patients in high and low risk according to a score obtained from the presence of different risk factors.47 The main advantages include simplicity and reproducibility.48 Of concern is the fact that the index has been validated prospectively in a small number of patients and only to predict the benefits of prophylactic antibiotics. Another simple index49 divides patients in four groups according to the original neutrophil (<3,100/μl) and lymphocyte (<1,500/μl) count. When both counts are lowest, the risk of neutropenic fever is the highest. This model was validated in patients receiving adjuvant chemotherapy of breast cancer with docetaxel, doxorubicin, and cyclophosphamide.
Among 266 patients with non-specified hematologic malignancies, Moreau et al.36 found that aggressive chemotherapy, baseline hemoglobin level, type and stage of disease, body surface area, involvement of the bone marrow, and monocyte count <150/μl were independent risk factors for neutropenic fever during the first course of chemotherapy. Among 240 patients with non-Hodgkin lymphoma, Pettengell et al.50 found that a model including age, weight, previous chemotherapy, chemotherapy dose, albumin levels, and use of granulocyte–colony-stimulating factor (G-CSF) predicted individual risk of neutropenic fever with 80% accuracy. While of interest, these models have limited use due to the fact that they involve only a small number of patients with specific malignancies.
In a prospective study of 3,760 patients treated for solid tumors and lymphomas in 115 practices in the United States, Lyman et al.30 were able to derive an individual risk of neutropenia, severe neutropenia, and neutropenic infection based on prior chemotherapy, low neutrophil count, abnormal hepatic and renal function, type of chemotherapy, and planned delivery of ≥85% of the dose intensity. The index has a predictive value positive and negative of 34% and 96%, respectively. Surprisingly, age was not an independent risk factor even though one-third of the patients were 65 and older. This model is interesting for several reasons. First, it was derived and validated from the cohort study of a large number of patients. Second, it included severe neutropenia in addition to neutropenic infections among the outcomes. Severe neutropenia should be avoided, at least in curable diseases, because it may purport a reduction of RDI of chemotherapy.51–54 Third, it provides a determination of individual risk of neutropenic infections. Fourth, it was derived from a number of community practices and as such it reflects the situation of the majority of patients undergoing cancer chemotherapy. Fifth, it encompasses a wide array of diseases and of chemotherapy regimens. The limits of this model include the need for a calculator and the fact that the age range of the patients was not specified. While we know that one-third of the patients were 65 and older, we don’t know how many of them were older than 75 or 80. In the time of electronic health records, the calculation of the index may not represent a problem anymore.
Due to the lack of prospective comparisons, it is premature to recommend a specific model for the prediction of neutropenic infections in individual cases. In a community-based oncology practice,55 the adoption of an instrument to assess the risk of neutropenic infections prior to the initiation of cytotoxic chemotherapy has led to a marked reduction in the incidence of neutropenic infections and of hospitalization for neutropenic infections. Also, more patients than before received chemotherapy with a RDI ≥85%.
Management of Neutropenia
Prevention of Neutropenic Infections in Patients Receiving Chemotherapy for Solid Tumors and Lymphomas
In the treatment of solid tumors and lymphomas, the management of neutropenia has two goals: reduction in the incidence and severity of infectious complications, and maintenance of the RDI of chemotherapy at ≥85%.
Prior to the introduction of the myelopoietic growth factors, it was recommended that the dose of chemotherapy be reduced in patients experiencing severe neutropenia at nadir or neutropenic infections and that the treatment be delayed for neutrophil counts ≤1,500/μl.2,22 Prophylactic administration of antibiotics has been a complementary strategy toward the reduction of neutropenic infections. The fluoroquinolones and the combination of sulfamethoxazole/thrimethrophrim reduce the risk of gram-negative infections from enteric pathogens in patients with prolonged neutropenia.56–59 By causing an anaerobe overgrowth in the colon, these compounds suppress the growth of gram-negative pathogens that are the most common cause of bacterial infections during neutropenia. Most of the studies were performed in patients with acute leukemia and in those undergoing bone marrow transplant, however, so that the information is limited in patients with solid tumors and lymphoma. In two large randomized controlled studies, prophylactic levofloxacin was associated with a reduction of episodes of neutropenic fever of approximately 30% without a reduction of documented infections or of infectious deaths.56,58 In one of these studies, stool cultures showed emergence of gram-negative organisms resistant to levofloxacin and rose the concern that this practice may lead to antibiotic-resistant hospital infections.56 According to a meta-analysis of randomized controlled trials, the prophylactic use of fluoroquinolones was associated with a significant reduction in the incidence of neutropenic fever in patients with solid tumors and lymphomas and a nonsignificant reduction in mortality.59 A Dutch study compared the combination of growth factors and antibiotics versus antibiotics alone in patients receiving treatment for small-cell lung cancer.60 The combination of colony-stimulating factors and antibiotics was more effective than antibiotic alone in preventing neutropenic fever. According to this study, the role of prophylactic antibiotics is questionable in the case of solid tumors.
Based on these data, no consensus exists on the use of prophylactic antibiotics in patients treated for solid tumors and lymphomas.61–63 This practice may be justified when the duration of neutropenia is expected to be longer than 7 days.
Myelopoietic Growth Factors
The introduction of recombinant hematopoietic growth factors (see Table 133.1) has provided a new weapon to prevent both neutropenia and neutropenic infections. Both recombinant G-CSF and granulocyte macrophage–colony-stimulating factor (GM-CSF) are available for clinical use.64–66 Recombinant forms of G-CSF include both short-acting (filgrastim, lenograstim, and tbo-filgrastim) and long-acting (pegfilgrastim and lipegfilgrastim) preparations. Unlike the short-acting preparations, pegfilgrastim and lipegfilgrastin cannot be eliminated from the kidneys. They are metabolized by the neutrophils at the time of recovery from neutropenia. As such, they linger in the circulation until recovery has occurred.
Three different recombinant human GM-CSFs are available and include sargramostim, molgramostim, and regramostim. Of these, sargramostim is the only one approved for use in the United States. Sargramostim is derived from yeast (Saccharomyces cerevisiae) and is O-glycosylated; molgramostim is not glycosylated recombinant human GM-CSF and is derived from Escherichia coli. Regramostim is fully glycosylated and is derived from Chinese hamster ovary cells. The different formulations appear to have similar pharmacologic activity.
Despite that they have been available now for more than 20 years, a number of controversies linger concerning hematopoietic growth factors. The controversies hinge on three questions: which growth factor should be used, when and how it should be used, and what are short- and long-term complications for their use.
Which Growth Factor Should be Used? In solid tumors and lymphomas, G-CSFs have reduced the incidence of neutropenia and neutropenic infections, as well as the hospitalization for neutropenic infections according to a number of randomized controlled studies examined in six meta-analyses8,67–71 and a review of its use in clinical practice in patients with lymphoma.5 In two of these meta-analysis,8,68 the use of these agents was also associated with reduced mortality from neutropenic infections. In addition, the most recent meta-analysis suggested that the all causes of mortality might have been reduced8 and that the dose intensity of chemotherapy was better preserved with these agents.9 At least one meta-analysis of 148 clinical trials failed to see any benefits for the use of myelopoietic growth factors.72 However, this study included different growth factors and different ways of administration such as delayed administration that was proven ineffective previously. According to a number of meta-analysis and systematic reviews, filgrastim and lenograstim have comparable activities.8,67,68,73 In a review of the drug approval process in Europe,74 the activity of the biosimilar Tevagrastim (Teva Pharmaceuticals, North Wales, PA) also appears comparable with that of filgrastim.
A number of randomized controlled studies found that safety and effectiveness of filgrastim and pegfilgrastim were comparable in patients with lymphoma and solid tumors.75–78 According to a meta-analysis of these trials, pegfilgrastim was superior to filgrastim in reducing the risk of neutropenic infections.70 Similar conclusions were reached by an analysis of a US claim database involving more than 18,000 patients79 and by a review of the use of filgrastim and pegfilgrastim in Spain.80 These results may be explained, at least in part by improved patient compliance with a single injection per cycle. In a randomized controlled trial, pegfilgrastim and lipegfilgrastim, the new long-acting form of filgrastim undergoing clinical trials, had comparable activities and safety.81
A number of studies demonstrated that GM-CSF decreases the duration of neutropenia and the risk of neutropenic infections in patients with lymphoma and solid tumors and is a reasonable choice for this purpose.61,82,83
At present, the studies comparing filgrastim and sargramostim provided inconclusive and somehow conflicting evidence of the superiority of one drug over the other. In a small randomized controlled study including 181 patients,84 filgrastim was associated with a shorter duration of neutropenia (1 day), but sargramostim was associated with decreased risk of hospitalization and shorter hospitalization for neutropenic fever. Both agents were equally well tolerated. A retrospective matched control study of national insurance claims indicated that the prophylactic use of sargramostim was associated with decreased risk of neutropenic infections and rehospitalization.85 In another study of similar design, pegfilgrastim proved superior to both filgrastim and sargramostim in preventing neutropenic infections and hospitalizations.86,87 According to another report,88 the substitution on the formulary of filgrastim with sargramostim in a single institution was associated with an increased incidence of infections and hospitalization rates as well as of treatment side effects (pain and fever). A review of 10 US outpatient practices revealed that sargramostim was associated with increased risk of complications including unexplained fever, fatigue, and skin eruptions.89 In a randomized comparison of filgrastim and sargramostim in patients with febrile neutropenia, sargramostim was also associated with increased risk of side effects.90 One may conclude that the efficacy of myeloid growth factors in use in the United States is comparable, sargramostim may have increased risk of side effects, and pegfilgrastim at present is the most practical and convenient of the growth factors.
Timing of Use. The questions related to the timing include primary versus secondary prophylaxis, treatment duration with filgrastim and sargramostim, the administration of pegfilgrastim on the same day of chemotherapy, and the effectiveness of growth factors in course of neutropenic infections.
Primary prophylaxis means treatment of patients at high risk of neutropenia or neutropenic infections beginning with the first cycle of chemotherapy treatment; in secondary prophylaxis, growth factors are reserved for those patients who had already experienced an episode of neutropenic fever.
A number of randomized controlled91–93 and retrospective94–96 studies demonstrated that primary prophylaxis decreased risk of neutropenic infections throughout treatment, not only during the first course of treatment, and therefore it should be preferred61,65 in patients at high risk.
Filgrastim and sargramostim are administered daily for a minimum of 7 to 10 days. It is common practice to discontinue filgrastim when the neutrophil count is ≥3,000/μl (though the drug insert recommends 10,000/μl). Several strategies have been employed for reducing the duration of growth factor treatment with inconclusive results. In a randomized controlled study, treatment of patients who had developed afebrile neutropenia did not reduce the risk of neutropenic infections.97 Another approach included the reduction of the administration of G-CSF to 5 days98; though it may be more effective than placebo, this approach may not be optimal. A review of several US practices revealed that administration of G-CSF for a duration shorter than 7 days was associated with increased risk of febrile infections and hospitalizations.99 As the risk of neutropenic infections is highest during the first two courses of chemotherapy, a study addressed the question of whether the use of filgrastim could be avoided after the first two chemotherapy cycles in patients receiving adjuvant treatment for early breast cancer.100 The risk of neutropenic infections was >20% during the successive chemotherapy cycles, and the authors concluded that filgrastim prophylaxis should be continued throughout all treatment cycles.
According to the current recommendations pegfilgrastim is administered once per treatment cycles between 24 and 72 hours from chemotherapy. These recommendations are based on the review of four randomized phase 2 studies including patients with breast cancer, lymphoma, nonsmall-cell cancer, and ovarian cancer.101 In the lymphoma and breast cancer studies, the duration of neutropenia was more prolonged and the neutropenia more severe in patients receiving same-day pegfilgrastim. Up to now, no new evidence has emerged supporting the use of pegfilgrastim on the same day as chemotherapy.
A meta-analysis of 13 randomized controlled studies of growth factors showed that myelopoietic growth factors do reduce the duration of hospitalization and the duration of neutropenia in patients with neutropenic infections.90 However, they do not affect the overall mortality, though they may have a marginal influence on infectious mortality. The same conclusions were reached by a more recent review of the literature.102 This use is not recommended in the current guidelines.61,65
Complications. The acute complications of growth factors include fever and skin reactions that appear more common with GM-CSF.90 Bone pain is particularly common with pegfilgrastim, generally occurs during the first 5 days of treatment, and may lead to discontinuation of the treatment.103
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








