THYROID-STIMULATING HORMONE STRUCTURE, BIOSYNTHESIS, AND FUNCTION
GLYCOPROTEIN HORMONE STRUCTURE
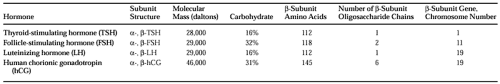
Thyroid-stimulating hormone (TSH, thyrotropin) is one of a family of glycoprotein hormones that also includes follicle-stimulating hormone (FSH), luteinizing hormone (LH), and human chorionic gonadotropin (hCG, Table 15-1). These hormones each consist of two dissimilar subunits, α and β, held together by strong noncovalent bonds.1 Each subunit consists of a polypeptide core that is stabilized by internal disulfide bonds and glycosylated at specific residues. The α subunits of all the glycoprotein hormones are identical and are highly conserved among different species. The β subunits of different hormones have extensive sequence homologies.
The glycoprotein hormones bind to membrane receptors in their target tissues. Hormonal specificities are determined by the respective β subunits. However, hCG and LH have weak intrinsic TSH bioactivity. The isolated α and β subunits are not bioactive. However, both α -TSH and β-TSH subunits contain receptor-binding domains. Modifications of amino-acid residues or of the carbohydrate side chains can result in TSH analogs with enhanced bioactivity or altered metabolic clearance.2
TSH has a molecular mass of ˜28 kDa. It is glycosylated at two sites on the α subunit and at one site on the β subunit. Glycosylation is required for subunit association, intracellular processing of the precursors to the secretory form of the hormone, and metabolic clearance of secreted hormone. Chemically deglycosylated TSH binds to its receptor but does not elicit a biologic response, suggesting that the glycosyl residues may have a role in receptor activation.3
The x-ray crystallographic structure of hCG is known,4 and a homologous structure for TSH has been proposed.5 The αand β subunits of the glycoprotein hormones share a common structural motif consisting of a central “cystine knot” formed by disulfide bonds, with two hairpin loops on one side of the knot and a single loop on the other. This cystine knot structure is found in growth factors and hormones, including platelet-derived growth factor, nerve growth factor, inhibins, and others.5 Another structural element presumed common to hCG, TSH, and the other glycoprotein hormones is a “seat-belt” region near the carboxyl terminus of the β subunit that wraps around the α subunit to maintain the heterodimeric structure.
BIOSYNTHESIS OF THYROID-STIMULATING HORMONE
Separate genes encode the α-TSH and β-TSH subunits.6 The human α gene is present in a single copy on chromosome 6 and is transcribed in all pituitary and placental cells synthesizing glycoprotein hormones. The human β-TSH gene is on chromosome 1. Hence, regulation of TSH gene transcription requires control of separate DNA regulatory elements for each subunit gene. Although all of the β subunits probably evolved from a common ancestor, β subunit genes are on separate chromosomes and do not form a single linkage group (see Table 15-1).
The α-TSH and β-TSH apoprotein cores are transcribed as prehormones, starting with leader sequences of hydrophobic amino acids that direct the nascent chains through the rough endoplasmic reticulum membrane (see Chap. 3). Pituitary glands contain excess α subunit relative to their β subunit content, and free subunits as well as intact hormone are secreted by the thyrotrope. Free α subunit generally can be detected in serum from euthyroid persons. It is increased in persons with primary hypothyroidism; free β-TSH also can be detected. Free α subunit (secreted by gonadotropes) also is increased in postmenopausal women. Free α subunit contains an additional oligosaccharide group that prevents it from combining with β-TSH.5
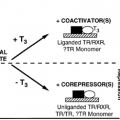
Thyrotropin-releasing hormone (TRH) stimulates α-TSH and β-TSH subunit gene transcription by inducing or activating specific transcriptional regulatory factors.7,8 Triiodothyronine (T3) causes a rapid fall in transcription of the α-TSH and β-TSH genes.9 The synthesis of β-TSH appears to be the rate-limiting step and a major regulatory point in the control of TSH. Regulatory regions involved in T3 suppression of subunit gene transcription (negative T3 response elements, TREs) have been identified for both the α-TSH and β-TSH genes.6 These negative TREs contain nucleotide sequences for interaction with the β receptor.10
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree