Thyroid disorders in children and adolescents
Introduction
Box 12-1 provides a classification of thyroid disorders in children. Thyroid system development, fetal thyroid physiology, thyroid dysfunction in premature infants, and congenital thyroid disorders (including inborn defects in thyroid hormone synthesis, metabolism, and action and thyroid binding protein abnormalities) are discussed in Chapter 6.
Thyroid hormones and their action
Few hormones exert as profound and essential role in human physiology as thyroid hormones.1,2 The major hormones released by the thyroid gland include tetraiodothyronine, or thyroxine (T4), and triiodiothyroine (T3).1 The production of these hormones involves several discrete biochemical steps that are depicted in Figure 12-1. Of these hormones, T3 plays the pivotal role in affecting physiology, being the molecule that principally binds to the thyroid hormone receptor (TRs). The thyroid hormone nuclear receptor belongs to the steroid hormone–retinoic acid receptor superfamily and is a regulator of DNA transcription.1,3,4 Two genes encode the TR; one on chromosome 17 designated alpha (TRa) and one on chromosome 3 designated beta (TRb).3,4 The TRs can exist as monomers or homodimers, and they can dimerize with other members of the family of nuclear receptors.3,4 After T3 binding to the TR, gene transcription is regulated in many tissues.4
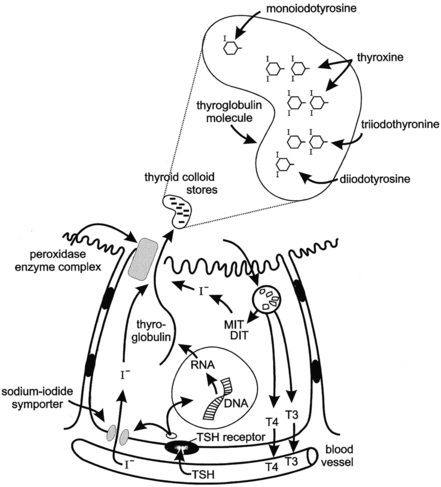
FIGURE 12-1  Illustration of thyroid hormone synthesis and secretion. TSH regulates the process via the G–protein-linked plasma membrane TSH receptor. TSH binding stimulates thyroglobulin synthesis and sodium-iodide symporter (iodide transporter) uptake of circulating iodide. Iodide diffuses in the cytosol to the apical membrane and is transported to the apical lumen by Pendrin, an anion-bicarbonate family exchanger, making iodide available to the enzyme organification complex (Pendrin; thyroid peroxidase, TPO, THOX). The tyrosine residues of thyroglobulin are iodinated at the apical cell membrane and are catalyzed by thyroid peroxidase, the organification enzyme. The resulting monoiodotyrosine (MIT) and diiodotyrosine (DIT) residues couple to form the iodothyronines thyroxine (T4) and triiodothyronine (T3) within the stored thyroglobulin molecule. TSH stimulates micropinocytosis of colloid droplets and progressive thyroglobulin proteolysis within the resulting phagolysosomes. T4 and T3 are secreted into the circulation. The uncoupled MIT and DIT are deiodinated by iodotyrosine deiodinase (DEHAL) to release iodide, which is largely recycled within the follicular cell. (From Fisher and Greuters [2008] Thyroid disorders in childhood and adolescence. In Pediatric Endocrinology, (3rd ed.) (pp 227-253). Philadelphia: Saunders.)
Illustration of thyroid hormone synthesis and secretion. TSH regulates the process via the G–protein-linked plasma membrane TSH receptor. TSH binding stimulates thyroglobulin synthesis and sodium-iodide symporter (iodide transporter) uptake of circulating iodide. Iodide diffuses in the cytosol to the apical membrane and is transported to the apical lumen by Pendrin, an anion-bicarbonate family exchanger, making iodide available to the enzyme organification complex (Pendrin; thyroid peroxidase, TPO, THOX). The tyrosine residues of thyroglobulin are iodinated at the apical cell membrane and are catalyzed by thyroid peroxidase, the organification enzyme. The resulting monoiodotyrosine (MIT) and diiodotyrosine (DIT) residues couple to form the iodothyronines thyroxine (T4) and triiodothyronine (T3) within the stored thyroglobulin molecule. TSH stimulates micropinocytosis of colloid droplets and progressive thyroglobulin proteolysis within the resulting phagolysosomes. T4 and T3 are secreted into the circulation. The uncoupled MIT and DIT are deiodinated by iodotyrosine deiodinase (DEHAL) to release iodide, which is largely recycled within the follicular cell. (From Fisher and Greuters [2008] Thyroid disorders in childhood and adolescence. In Pediatric Endocrinology, (3rd ed.) (pp 227-253). Philadelphia: Saunders.)
T4 is the predominant hormone released from thyroid follicular cells. After release it circulates in protein-bound and free states at a ratio of about 1000 to 1. Thyroid hormone-binding proteins in the blood include thyroxine-binding globulin (TBG), prealbumin or transthyretin, and albumin.5–7 TBG is the predominant carrier protein for T4; TBG and albumin also carry T3.5–7 In the euthyroid steady, the circulating concentration of free T4 (FT4) and free T3 are about 0.03% and 0.30%, respectively, of total hormone concentrations.
It is important to recognize that circulating levels of thyroid hormones and carrier proteins change with age (Tables 12-1 and 12-2). Absolute mean free T4 and free T3 concentrations are about 10 and 4 pg/mL, respectively, and differ according to age. In adolescents and adults, the plasma concentrations of the several binding proteins are 1 to 3 mg/dL for TBG, 20 to 30 mg/dL for TBPA, and 2 to 5 g/dL for albumin.2,5–7 TBG concentrations are greater in children than in adults, and they decline to adult levels during adolescence.2 Because the thyroid-hormone binding proteins are produced in the liver, they are acute phase reactants, with concentrations increasing during acute illness2,5–7; they also increase in response to estrogen exposure.
TABLE 12-1
Changes with Age in Serum Concentrations of T4, TSH, TBG, and Thyroglobulin (Tg)
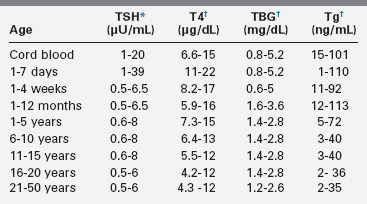
†Mean and two standard deviations (SD) range.
Compiled from Fisher, D. A., & Vanderschueren-Lodeweycky, M. (1985). Laboratory tests for thyroid diagnosis in infants and children. In F. Delange, D. A. Fisher (Eds.), Pediatric thyroidology (pp. 127–142). Basel: Karger; Walfish, P. G., & Tseng, K. H. (1989). Thyroid physiology and pathology. In R. Collu, J. R. Ducharme, H. Guyda (Eds.), Pediatric endocrinology (pp. 367–448). New York: Raven; Delange, F., Dahlem, A., Bourdoux, P., et al (1984). Increased risk of primary hypothyroidism in preterm infants. Pediatrics, 105, 462; Pazzino, V., Filetti, S., Belfiore, A., et al. (1981). Serum thyroglobulin levels in the newborn. J Clin Endocrinol Metab, 52, 3634; Delange, F. (1993). Thyroid hormones: biochemistry and physiology. In J. Bertrang, R. Rappaport, P. C. Sizonenko (Eds.), Pediatric endocrinology (pp. 242–251). Baltimore: Williams and Wilkins; Lazar, L., Frumkin, R. B., Battat, E., et al. (2009). J Clin Endocrinol Metab, 94, 1678–1682.
TABLE 12-2
Changes with Age in Serum Concentrations of T3, rT3, Free T4, and Free T3
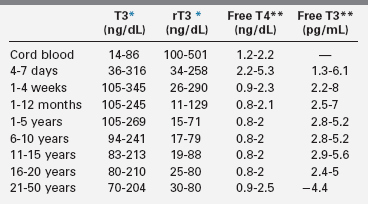
*Geometric mean and range.
†Two standard deviations (SD) range, by tracer dialysis.
Compiled from Delange, F. (1993). Thyroid hormones: biochemistry and physiology. In J. Bertrang, R. Rappaport, P. C. Sizonenko (Eds.), Pediatric endocrinology (pp. 242–251). Baltimore: Williams and Wilkins; Lucas, C., Carayan, P., Bellhilehi, J., & Giraud, F. (1980). Changes in levels of free thyroid hormones in children from 1 to 16 years: comparison with other thyroid indices. Pediatric, 35, 197; Nelson, J. C., Clark, S. J., Borut, D. L., et al. (1993). Age related changes in serum free thyroxine during childhood adolescence. J Pediatr, 123, 899.
Conversion of T4 to T3 involves the deiodination of T4 (Figure 12-2). Monodeiodination of the beta or outer ring by monodeiodinase (MD) type II produces T3.8,9 Monodeiodination of the alpha or inner-ring produces reverseT3 (rT3), which is inactive metabolically. Under normal circumstances, T3 and rT3 are produced at similar rates. About 70% to 90% of circulating T3 is derived from peripheral conversion of T4, and 10% to 30% of circulating T3 is from the thyroid gland.2 Reflecting the age-related changes in the hormones that regulate T4 stability, the clearance of T4 generally decreases from infancy to adulthood (Table 12-3).
TABLE 12-3
Variation in Peripheral Thyroxin Metabolism with Age*
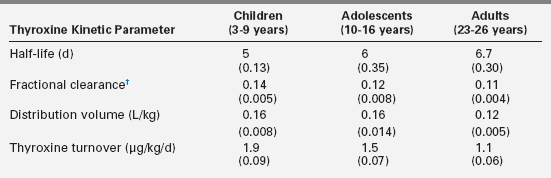
†Fraction of extrathyroid pool per day.
Data from Beckers, C., Malvaux, C., & De Visscher, M. (1966). Quantitative aspects of the secretion and degradation of thyroid hormones during adolescence. J Clin Endocrinol Metab, 26, 202–306; Sterling, K., & Chodos, R. (1956). Radiothyroxine turnover studies in myxedema, thyrotoxicosis and hypermetabolism without endocrine disease. J Clin Invest, 35, 806–813.
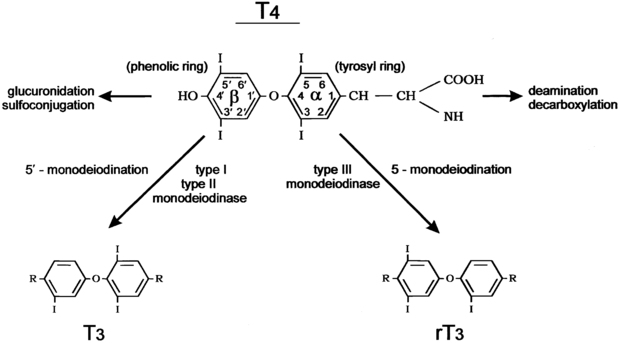
FIGURE 12-2  The metabolism of thyroxine (tetraiodothyronine). The major metabolic pathway is progressive monodeiodination mediated by the three iodothyronine monodeiodinase enzymes type I, type II, and type III. Outer (phenolic) ring 5’monodeiodination produces active 3,5,3’ triiodothyronine. Inner (tyrosyl) ring 5’monodeiodination produces inactive reverse 3,3,5’ triiodothyronine. Type I deiodinase is also capable of inner-ring monodeiodination. The alanine side chain of the tyrosyl ring is also subject to degradative reactions, including deamination and decarboxylation. Sulfoconjugation and glucuronide conjugation reactions at the 4’ phenolic ring site occur largely in liver tissue. (From Fisher and Greuters [2008] Thyroid disorders in childhood and adolescence. In Pediatric Endocrinology, (3rd ed.) (pp 227-253). Philadelphia: Saunders.)
The metabolism of thyroxine (tetraiodothyronine). The major metabolic pathway is progressive monodeiodination mediated by the three iodothyronine monodeiodinase enzymes type I, type II, and type III. Outer (phenolic) ring 5’monodeiodination produces active 3,5,3’ triiodothyronine. Inner (tyrosyl) ring 5’monodeiodination produces inactive reverse 3,3,5’ triiodothyronine. Type I deiodinase is also capable of inner-ring monodeiodination. The alanine side chain of the tyrosyl ring is also subject to degradative reactions, including deamination and decarboxylation. Sulfoconjugation and glucuronide conjugation reactions at the 4’ phenolic ring site occur largely in liver tissue. (From Fisher and Greuters [2008] Thyroid disorders in childhood and adolescence. In Pediatric Endocrinology, (3rd ed.) (pp 227-253). Philadelphia: Saunders.)
Regulation of thyroid function
The production of T4 and T3 within the thyroid gland is regulated by the thyroid stimulating hormone (TSH; also called thyrotropin), which is released from the anterior pituitary gland (Figure 12-3).10,11 TSH receptors are present on thyroid follicular cells and are G protein–coupled receptors with a large extracellular amino terminus.12 Mutations of the TSH receptor can result in constitutive activation of the receptor with severe hyperthyroidism, whereas inactivating mutations result in TSH unresponsiveness and hence hypothyroidism.12
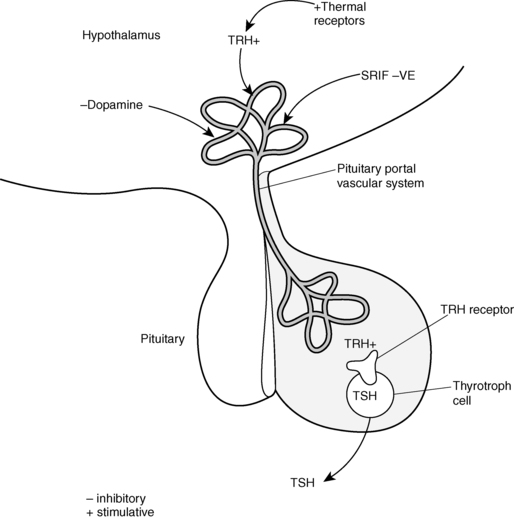
FIGURE 12-3  The hypothalamic-pituitary TSH axis. Thyrotropin-releasing hormone (TRH) secreted into the pituitary portal vascular system stimulates TSH synthesis and secretion from the pituitary thyrotroph cell. TRH secretion is modulated by central and peripheral thermal sensors. Dopamine or somatostatin (SRIF) can inhibit TSH release. (From Fisher and Greuters [2008] Thyroid disorders in childhood and adolescence. In Pediatric Endocrinology, (3rd ed.) (pp 227-253). Philadelphia: Saunders.)
The hypothalamic-pituitary TSH axis. Thyrotropin-releasing hormone (TRH) secreted into the pituitary portal vascular system stimulates TSH synthesis and secretion from the pituitary thyrotroph cell. TRH secretion is modulated by central and peripheral thermal sensors. Dopamine or somatostatin (SRIF) can inhibit TSH release. (From Fisher and Greuters [2008] Thyroid disorders in childhood and adolescence. In Pediatric Endocrinology, (3rd ed.) (pp 227-253). Philadelphia: Saunders.)
TSH release is regulated by the hypothalamic hormone thyrotropin releasing hormone (TRH) (see Figure 12-3 ).13,14 This peptide hormone is produced in medial neurons of the paraventricular nucleus of the hypothalamus and is released into the portal circulation of the pituitary gland.13,14 Several different neurotransmitters have been observed to influence THRH release.15,16
In addition to normal regulation of TSH receptor activity by TSH, thyroid function can be adversely affected by antibodies that can either stimulate or block TSH action. TSH receptor–stimulating immunoglobulin antibodies (TSI), or thyroid receptor antibodies (TRAb), are present in the circulation of individuals with Graves disease and activate TSH receptors.17–19 Conversely, TSH receptor–blocking immunoglobulins (TBI) antagonize TSH action and can lead to hypothyroidism.17–19
Clinical and biochemical assessment of thyroid status
Clinical evaluation of thyroid function
Thyroid disease can present with overt symptoms, insidiously, or with isolated thyromegaly. Thus, evaluation of the thyroid gland should be included in the routine examination of children. The thyroid gland can be visualized by having the patient look to the ceiling and swallow. As the thyroid moves, the margins of the gland are viewed to estimate size and symmetry. The thyroid should be palpated to assess size, consistency, and symmetry. This is best performed with the clinician standing behind the patient and palpating the neck with the fingertips. The texture of the thyroid can be assessed to determine if it is smooth or irregular, and the texture may feel firm or soft if nodules are present. If any asymmetry or abnormal thyroid fullness is noted, ultrasonographic evaluation is recommended, as pathologic thyroid nodules may feel like normal tissue.
To assess gland size, one may estimate the size of each thyroid lobe relative to that of a teaspoon (5 g) or a tablespoon (15 g). Generally, until the end of puberty, gland size (in grams) approximates the patient’s age in years times 0.5 to 0.7.20 Thus, each thyroid lobe of a 10-year-old is approximately one half of a teaspoon for a total gland size of 5 to 7 g.20 For teens and adults, each lobe of the thyroid may reach one teaspoon in size for an approximate total gland size of about 10 g.20
When a sublingual thyroid gland is discovered late in childhood or in adolescence, the tissue should be palpated with a gloved finger during regular office visits, because nodules and malignancies may develop in ectopic thyroid glands.21 In contrast, when an ectopic thyroid is detected in infancy and replacement therapy is initiated, the residual thyroid tissue becomes atrophic and does not present long-term problems.
Biochemical evaluation of thyroid function
Thyroid function can be assessed by measurement of total T4 and T3 levels, along with indices that reflect thyroid hormone-binding proteins (T3 or T4 resin uptake).22 The levels of estimated free (unbound) T4 (FT4) are measured to assess thyroid hormone status without the confounding influences of carrier proteins.
Several conditions occur in which thyroid hormone levels are abnormal, yet the individual is euthyroid. Because of their confusing nature, these conditions may result in the patient being erroneously diagnosed or treated for hypothyroidism or hyperthyroidism. When FT4 values are normal yet total T4 values are high, familial dysalbuminemic hyperthyroxinemia needs to be considered as the cause.23,24 In the United States, this autosomal dominant disorder is most commonly seen in Hispanic individuals and can be diagnosed by thyroid hormone–binding protein electrophoresis. If FT4 values are normal but total T4 values are low, the possibility of TBG deficiency must be entertained. TBG deficiency is an X-linked disorder that may be associated with color blindness.25 In these and other conditions affecting thyroid hormone binding, treatment is not needed and the patient should be educated about the condition to avoid unnecessary treatment by unsuspecting practitioners.
As indicated previously, T4 is much more abundant in the circulation, but T3 is the more metabolically active thyroid hormone. The majority of T3 is produced peripherally from T4, but some (10% to 30%) is also secreted by the thyroid gland. A metabolically inactive form ofT3, reverse T3, also is produced, and its concentration is elevated in conditions such as euthyroid sick syndrome26 (see Figure 12-2). Ultrasensitive thyrotropin or thyroid stimulating (TSH) assays have been developed, and assessment of TSH has greatly improved the evaluation of thyroid status.27 TSH concentrations help to distinguish many thyroid disorders that present with either low or high T4 concentration. TSH values within the normal range for the assay are indicative of a euthyroid state if the hypothalamic pituitary axis is intact. Elevations of TSH above the normal levels for age generally indicate primary thyroid hypofunction; suppressed or undetectable TSH values generally indicate hyperthyroidism, providing that there are no substances in the circulation such as drugs or endogenous antibodies that interfere with the assay. When both FT4 and TSH levels are elevated, TSH-producing pituitary adenomas or thyroid hormone resistance needs to be considered.
Critical in the interpretation of thyroid hormone concentration is the recognition that concentrations of T4, T3, and TSH vary with age (see Box 12-1). TSH values in children differ from those in adults, defined by an upper limit value of about 4 μU/mL or less.28–31 In comprehensive studies of this issue, the upper limit of TSH values in healthy children and adolescents without thyroid disease is about 7μU/mL.32–34 The application of an adult reference range to children results in the erroneous diagnosis of subclinical hypothyroidism and the unnecessary referral of children for subspecialty care by primary care providers.
Hypothyroidism
The public and many practitioners commonly believe that hypothyroidism is associated with and is a cause of obesity, yet there is little support for the notion that the hypothyroid state contributes to obesity.35,36 It is also important to note that TSH levels are slightly higher in obese individuals than nonobese individuals.37–40 With weight loss, TSH levels normalize in these children.37–39,41 Thus, slight elevations in TSH in obese individuals are physiologic, reflecting an attempt by the body to increase metabolism and limit adipose tissue deposition and, hence, do not warrant therapy.
The most common causes of hypothyroidism in children are autoimmune processes resulting in Hashimoto thyroiditis.42,43 Autoimmune thyroiditis also leads to juvenile acquired hypothyroidism that can present with growth failure when chronically present.44 Hypothyroidism in children can be caused by iodine exposure or hypothalamic-pituitary dysfunction. Other causes of hypothyroidism include exogenous goitrogens,45,46 cystinosis,47,48 acute and subacute thyroiditis,49 and thyroid irradiation during cancer treatment.50 Hypothyroidism in the newborn is a serious health concern and is detected by newborn screening programs as detailed in Chapter 7
Hashimoto or autoimmune thyroiditis
Autoimmune thyroiditis with thyroid enlargement is one of the most common presentations of childhood thyroid disease.43,51 It is associated with antibodies against thyroglobulin and thyroperoxidase and is characterized by lymphocytic infiltration of the thyroid gland, which results in thyromegaly.43,51 Depending on the nature of the antithyroid antibodies, Hashimoto disease may be associated with a euthyroid state, hypothyroidism, or transient hyperthyroidism.43,51 Damage to the thyroid gland reflects both antibody-mediated and cell-mediated injury.
Hashimoto thyroiditis may rarely occur in very young infants52 but typically presents in adolescents, affecting females three to five times more commonly than males.43 The thyroid gland is usually diffusely enlarged and may seem to have an irregular, cobblestone texture on palpation. Asymmetric thyroid enlargement, mimicking a thyroid nodule, may be noted. The presence of antithyroid antibodies and the absence of nodules on ultrasonography can distinguish inflammation from other pathologic processes.
Importantly, the presence of antithyroid antibodies does not portend the development of complete or partial thyroid failure that will warrant therapy. In the healthy adult population, up to 5% of individuals had circulating antithyroid antibodies present.53 Less than 10% of these individuals (i.e., only 0.5% of those with antibodies) will develop hypothyroidism, and those who have elevated antithyroperoxidase (TOP) antibodies are much more at risk than those who have antithyroglobulin (TG) antibodies.53
In children, the incidence of antithyroid antibodies in the population is not defined. Of those children with antithyroid antibodies, about 20% are reported to develop hypothyroidism that requires treatment with exogenous thyroid hormone,54,55 and these children often have very high antithyroid antibody titers. If a child is found to have low concentrations of antithyroid antibodies, it is reasonable to assess thyroid indices every 6 to 12 months and initiate therapy when the TSH rises above the upper limit of normal for children. If high titers are present at presentation, it is reasonable to initiate therapy at that time.
In some children, untreated Hashimoto thyroiditis can result in progressive thyromegaly and hypothyroidism.43 Treatment with levothyroxine prevents hypothyroidism and the TSH elevations that stimulate gland enlargement. When T4 levels are modestly depressed (< 5 μg/dL) or normal, treatment can be initiated with 1 to 2 µg/kg/day of levothyroxine. If profound hypothyroidism is present, pseudotumor cerebri may develop when children are treated with conventional doses.56 Thus, treatment is initiated with one third to one half of the usual dose of levothyroxine. After 2 to 4 weeks, the patient can be advanced to conventional doses. However, children with profound hypothyroidism can develop pseudotumor cerebri even if treatment is initiated with low doses of levothyroxine.57–59
It has been reported that some children with profound TSH elevations (> 250 mIU/mL) and those with severe hypothyroidism may experience complete spontaneous resolution of the hypothyroid state without treatment and restoration of a euthyroid state occurs.60 Based on the experience of others, this is very uncommon.
Although it has been reported that there are some differences in the oral bioavailability of different levothyroxine preparations,61–63 from a practical vantage these differences are minor.62–64 Thus, the routine use of less expensive generic compounds versus more expensive brand-name products is justified.
The timing of levothyroxine ingestion has been the subject of study. In contrast to the standard recommendation that thyroid replacement be taken on an “empty stomach,” taking the medication at bedtime is associated with higher T4 levels and lower TSH levels over the course of the day.65,66 This is believed to be related to better gastrointestinal absorption in the evening than during the day.65,66
The suggestion has also been made that hypothyroidism in adolescents can be treated with a single dose given weekly.67 This approach is not recommended, because thyroid hormone levels are high shortly after the dose is administered and are low by the week’s end.67 Treatment of congenital hypothyroidism with weekly doses of levothyroxine can result in mental retardation.68 It is also recognized that excessive soy intake, iron tablets, and excessive fiber can interfere with the absorption of levothyroxine.69–71
Other potential therapies that may theoretically alter the autoimmune process have been tested. Among the proposed remedies, no benefit has been observed in patients who have taken selenium.72
Hashitoxicosis
Uncommonly, patients may present with hashitoxicosis, in which the immunologic destruction of thyroid tissue results in the release of preformed thyroid hormone, leading to elevated T4 levels in the circulation with symptoms and signs of hyperthyroidism.73 In contrast to Graves disease, hyperthyroidism is transient, eye findings are absent, radionuclide uptake is low, and levels of thyroid-stimulating immunoglobulins are not elevated.73
Hashimoto thyroiditis may be associated with other autoimmune diseases, including diabetes mellitus, adrenal insufficiency, vitiligo, and hypoparathyroidism.74 Autoimmune thyroiditis is also seen in patients with inflammatory bowel disease and juvenile arthritis.75,76 Annual surveillance of thyroid gland size and TSH levels should thus be considered for children with other autoimmune problems, and clinicians should be vigilant for signs of hyperthyroidism or hypothyroidism. Conversely, children with autoimmune thyroiditis should be observed for signs of diabetes mellitus and Addison disease (see Chapter 20 for a discussion of autoimmune polyglandular syndromes).
The incidence of coexisting celiac disease in the setting of Hashimoto thyroiditis is about 1%.77 If patients manifest abdominal discomfort, weight loss, or gastrointestinal symptoms, celiac disease screening should be performed, but does not need to be done routinely in children with thyroid disease. We have found a 1% incidence of autoimmune liver disease in children with autoimmune thyroid disease. Because such liver disease can be occult, we annually assess circulating transaminase values (alanine amino transferase [ALT] and aspartate amino transferase [AST]). If values are elevated, evaluation of possible autoimmune liver disease is initiated and level of antineutrophil antibodies (ANA) as well as smooth muscle and liver-kidney microsomal antibodies obtained.78
Several groups of children are at risk for autoimmune thyroiditis. Because girls with Turner syndrome are predisposed to autoimmune thyroiditis,79 TSH levels should be assessed annually. Turner syndrome should be considered in girls with hypothyroidism, especially if the child is prepubertal at presentation.80,81 Children with Down syndrome warrant annual screening for hypothyroidism because they are more prone to develop autoimmune disorders.82,83
Subclinical hypothyroidism
Subclinical hypothyroidism refers to a situation where circulating T4 and T3 concentrations are normal but TSH values are elevated.36,84 As noted previously, many children are erroneously diagnosed with this condition when TSH concentrations are found to be elevated relative to adult reference range values.33 However, if appropriate pediatric-based TSH values are applied, the majority of children so diagnosed will not have hypothyroidism. Thus, some experts have questioned if subclinical hypothyroidism is a real entity in children.36,84
Studies of children with mild TSH elevations (5 to 10 μU/mL) reveal that only a small fraction will progress to TSH elevations > 10 μU/mL.36,84 Data also show that treatment of children with TSH values of 5 to 10 μU/mL do not exhibit somatic or other benefits when treated with levothyroxine.36,84 Thus, treatment of children with TSH levels < 10 μU/mL is not needed. For children with TSH levels > 10 μU/mL, treatment with low doses of levothyroxine is indicated.
Juvenile acquired hypothyroidism
When autoimmune thyroiditis occurs during childhood, it is referred to as juvenile acquired hypothyroidism. In children, severe hypothyroidism can be well tolerated. Thus, prolonged hypothyroidism may not be detected until growth failure occurs.44,85
Because untreated infantile hypothyroidism is associated with mental retardation, the assumption is often made that juvenile hypothyroidism is associated with learning problems and poor academic performance. This notion is not correct, as children with juvenile hypothyroidism can be successful academically and do not manifest overt learning problems or cognitive impairment related to the hypothyroid state,
Children with severe hypothyroidism may manifest cold intolerance, decreased frequency of bowel movements, and decreased physical activity.44,85 Bradycardia, facial puffiness, delayed reflexes, and carotenemia may be present. In comparison with Hashimoto thyroiditis, the thyroid gland is either small or only modestly enlarged. 44,85 Antithyroid antibodies usually are present.44,85 These patients are generally not obese, and body mass index values are similar before and after treatment.35,86 The development of slipped capital femoral epiphyses may antedate the detection of hypothyroidism.87
Some children with juvenile hypothyroidism may present with signs of puberty but without pubic hair.88–90 Boys may present with testicular enlargement and girls may present with menarche, with or without breast development.91 With treatment of the hypothyroid state, these characteristics may regress.88–90 Available evidence suggests that the hypothyroid state leads to increased gonadotropin secretion, which triggers gonadal activity.88,91 Alternatively, it has been suggested that extreme high levels of TSH cross-react with the follicle-stimulating hormone (FSH) receptor in the gonads. This entity is referred to as the “overlap” or Van Wyk–Grumbach syndrome.92,93 The very high TSH values reflect pituitary hyperplasia of thyrotrophs and may be associated with an appearance of an enlarged pituitary on imaging studies that might be mistaken for a pituitary TSH secreting adenoma.94 However, these findings resolve with treatment of the hypothyroid state. In some children, true puberty may develop within a year or two of treatment onset, which may limit catch-up growth due to earlier closure of epiphyses induced by sex steroids.44
Juvenile hypothyroidism may not be recognized until a sizable statural deficit is present, and the lost height is usually not recovered.44 Children with juvenile hypothyroidism who present with growth failure manifest very low T4 values that are often less than 2 μg/dL and profoundly elevated TSH levels that are higher than 250 μU/mL.44 Hypercholesterolemia and anemia may be present.44
The magnitude of the height deficit is proportional to the duration of hypothyroidism, which can be estimated as the difference between the chronologic and bone age.44 When the individual is treated with conventional doses of levothyroxine, accelerated skeletal maturation is observed, with the skeletal age advancing disproportionately faster than gains in height.44 Thus, predicted heights fall, and genetic growth potential is not achieved.
Because of the potential poor growth outcomes of patients with hypothyroidism, we have treated these patients with low doses of levothyroxine (0.25 to 0.5 μg/kg/day; e.g., 50 μg for a 10-year-old). With this regimen we find that, with low-dose levothyroxine therapy, T4 values normalize (6 to 7 μg/dL) within 2 months, and TSH levels normalize or remain only modestly elevated. Moreover, serial bone age determinations do not show the disproportionate advancement of skeletal age seen with conventional therapy. However, we do not know if this approach leads to more favorable height outcomes.95 Some physicians have also suggested that treating these children with gonadotropin-releasing hormone analogs to delay puberty will lead to improved long-term growth.96–100 However, we have found that catch-up growth slows markedly in some hypothyroid children receiving gonadotropin-releasing hormone analog therapy so that predicted adult heights is less and others have not observed added benefit.101 Because the loss in adult height is proportional to the duration of hypothyroidism,44 early detection of this disorder is the best intervention for preventing statural deficits.
Iodine-induced hypothyroidism
Sixty percent of the weight of T4 is iodine, and iodine is the rate-limiting substrate for synthesis of thyroid hormones2 (see Figure 12-1). Iodine is present in small amounts (15 to 20 mg) in humans. The recommended dietary allowance of iodine is 100 μg/day for adolescents and adults, and 150 μg/day for pregnant and lactating women. It is 60 to 100 μg/day for children aged 1 to 10 years, 40 μg/day for infants aged 6 to 12 months, and 30 μg/day for infants 6 months of age or younger. In areas of low iodine intake, the recommended dietary intake should be 90 μg for infants aged less than 1 year.2
Although modest iodine intake is essential for thyroid function, high-level iodine exposure results in an acute block in the release of preformed thyroid hormone and impaired thyroid hormone synthesis, a phenomenon referred to as the Wolff-Chaikoff effect.102 When iodine-induced hypothyroidism is suspected, it can be diagnosed by the detection of high iodine levels in urine samples.103
In children, iodine can be absorbed through the skin, and iodine-induced hypothyroidism has been observed after cutaneous iodine or Betadine use.103–105 Iodine-induced suppression of thyroid hormone production has also been observed in children with central intravenous lines when regular cleansing of the insertion site with iodine was included in central line care. Neonatal hypothyroidism has also been associated with maternal povidone (iodine) exposure at the time of delivery.104
In preterm infants, iodine-induced hypothyroidism warrants special attention, because the suggestion has been made that cutaneous iodine exposure is a major cause of hypothyroidism in premature infants.106 Studies show that iodine-induced hypothyroidism is infrequent in the United States.107
Significant iodine exposure also occurs from amiodarone, an antiarrhythmic drug that contains 37% iodine.108 Hypothyroidism occurs in 10% of individuals treated with this compound.109 Amiodarone can also reach the fetus by transplacental passage and induce fetal hypothyroidism.108
In addition to iodine excess, iodine deficiency also leads to hypothyroidism. Estimates indicate that more than 1 billion people worldwide are at risk for iodine deficiency.110 Clinically, iodine deficiency is associated with goiter, hypothyroidism,110 and endemic cretinism.111 Endemic cretinism is classified into neurologic or myxedematous type,112 with severe mental retardation, mutism, and cerebral diplegia found in neurologic type. Children with the myxedematous type, have less severe mental retardation and have severe growth retardation and myxedema.112
Geographic areas of iodine deficiency exist, even in the United States.113,114 With the prevalent use of iodized salt, however, the incidence of iodine deficiency has been markedly reduced, and hypothyroidism and goiter due to iodine deficiency are rare in the developed world.113,114 Iodine intake in the United States has declined, an issue that may have future clinical implications.113,114 In Australia, a reduction in iodine intake has been reported, with potential implications for pregnant and lactating women,115 as reduced iodine intake may predispose to maternal and child hypothyroidism predisposing to hypothyroidism or developmental delay.112 The exclusive use of deiodized salt, which includes sea salt, is thus not recommended.
Hypothalamic-pituitary dysfunction
Central hypothyroidism should be considered in children with a history of head trauma, brain tumors, meningitis, central nervous system irradiation, or congenital nervous system malformations. Central hypothyroidism has also been associated with the use of retinoid X receptor-selective ligands in the treatment of lymphomas.116
In contrast to primary hypothyroidism, the diagnosis of hypothyroidism secondary to hypothalamic-pituitary dysfunction may be difficult to establish. Often, circulating concentrations of T4 are in the low-normal range, and TSH may be low, normal, or elevated.117,118 FT4 values, however, are usually low.
Whereas congenital central hypothyroidism will be diagnosed in states that perform T4 screening of newborns, neonatal screening programs that rely on TSH determinations will not detect this condition. Central hypothyroidism should therefore be suspected in infants with cholestasis, poor growth, hypoglycemia, structural nervous system problems, or pituitary insufficiency.119 When neonatal T4 values are interpreted, care should also be taken to use infant thyroid hormone values for comparison, because infantile T4 levels are higher than those seen in adults120 (see Tables 12-1 and 12-2; also see Chapter 7).
Importantly, up to 30% of children who will develop central hypothyroidism may have normal T4 and TSH levels at birth.121 Thus, all children with evidence of hypopituitarism should be regularly monitored for central hypothyroidism onset.
When central hypothyroidism is suspected, the thyrotropin releasing hormone (TRH) test helps distinguish pituitary (secondary) and hypothalamic (tertiary) hypothyroidism.118,120,122 Typically, there is a minimal rise in serum TSH levels in response to TRH in patients with pituitary disease, whereas there is a delayed response (> 60 minutes) in patients with hypothalamic disease. However, responses to this test are variable, making it difficult to distinguish between pituitary and hypothalamic hypothyroidism.118,120,122 Central nervous system imaging should be performed to look for congenital malformations or hypothalamic-pituitary lesions. Care should be taken to search for other pituitary hormone deficiencies, especially abnormalities of the hypothalamic-pituitary adrenal and growth hormone axes, as gene defects that adversely affect hypothalamic pituitary dysfunction, include mutations in the LIM/homeobox protein 3 (LHX3) and 4, prophet of pit-1 (PROP1), and pituitary transcription factor (PIT).123–125
Treatment consists of replacement therapy with levothyroxine. Some children with central hypothyroidism require doses lower than those used to treat primary hypothyroidism.126 Because TSH values are not helpful in guiding treatment, measurement of FT4 levels is recommended.126 Furthermore, a dose of 1.6 μg/kg of levothyroxine is recommended to maintain the FT4 levels in the upper half of the reference range.127
Giant hemangiomas
Hypothyroidism has been associated with giant hemangiomas.128 In some infantile hemangiomas, the endothelium of these vascular structures produces type 3 iodothyronine deiodinase, which degrades circulating T4 (see Figure 12-2). Treatment of hypothyroidism in this setting requires high doses of levothyroxine.128
Hypothyroidism in cancer survivors
It is well recognized that children who are cancer survivors and who have had head and neck irradiation are at increased risk for differentiated thyroid cancer.129,130 More common, though, is the development of mild hypothyroidism.131–133 Up to 30% of children who have had head and neck irradiation will develop primary hypothyroidism.133 Thus, annual TSH screening is suggested. In addition, ultrasound studies are recommended beginning 5 years after radiation exposure.
Practitioners who argue that palpation alone is sufficient for the follow-up of individuals who have had head and neck irradiation need to recognize that ultrasonography will detect thyroid nodules well before palpation.130,134 It must also be recognized that earlier rather than later recognition of thyroid cancer can lead to less extensive surgery, lower administered activities of 131I, and a better chance of cure.135
Thyroid hormone resistance
Thyroid hormones exert their effects by binding to a specific nuclear receptors to regulate cellular gene expression.136 When the thyroid hormone receptor is mutated, impaired tissue responsiveness results, leading to thyroid enlargement, elevated levels of T4 and T3, tachycardia, and behavioral problems.137–140 Unlike Graves disease, TSH values are normal or slightly elevated.
The most common forms of thyroid hormone resistance are caused by mutations of the thyroid hormone receptor beta gene.138–140 More than 100 mutations have been identified that result in impaired affinity for T3.138 Mutant thyroid hormone receptors also block the function of normal thyroid hormone receptors.138 Thus, thyroid hormone resistance is a dominant-negative mutation, and inheritance is autosomal dominant.138 Detection of thyroid hormone resistance in the index case may therefore lead to diagnosis of the condition in other family members. In up to 50% of children with thyroid hormone resistance, the mutations are spontaneous.
Most individuals with resistance to thyroid hormone have generalized thyroid hormone resistance.138 These individuals are eumetabolic and asymptomatic, with TSH levels in the normal range.
In contrast, some individuals have isolated pituitary thyroid hormone resistance. These individuals have symptoms of hyperthyroidism, because they are sensitive to the effects of increased circulating thyroid hormone in the periphery, where the thyroid receptors are functionally intact.141 Resistance to thyroid hormone can be associated with central nervous system problems. Approximately 50% of individuals with resistance to thyroid hormone have attention deficit hyperactivity disorder and a minority have mental retardation.142
Because individuals compensate for thyroid hormone resistance by secreting more thyroid hormone, treatment is generally not necessary.138,143 However, some patients with thyroid hormone resistance may be improperly diagnosed as having Graves disease and undergo ablation of the thyroid. In this situation, replacement therapy with appropriate doses of exogenous thyroid hormone is needed. With the earlier recognition of resistance to thyroid hormone due to newborn screening, the issue of whether children with resistance to thyroid hormone should be treated prenatally or during infancy has been raised.138,143 Treatment is generally reserved for infants who show elevated TSH levels, growth failure, seizures, and developmental delay.138,143
In some cases, TSH secretion may be profound, leading to massive thyromegaly, which may adversely impact upper airway function.144,145 These cases are associated with severe loss of function mutations. Treatment with high doses of triiodiothyroine every other day has been shown to be somewhat effective in this setting.144,145 In other cases, thyroidectomy is needed to prevent airway compromise.144,145
Hyperthyroidism
Hyperthyroidism occurs less commonly in children than hypothyroidism, yet is far more symptomatic.146–150 Graves disease is the most common cause of childhood thyrotoxicosis and is characterized by diffuse goiter, hyperthyroidism, and occasionally ophthalmopathy. Other causes of hyperthyroidism in children include autonomously functioning thyroid nodules, neonatal thyrotoxicosis, and infections of the thyroid. Hyperthyroidism also results from thyroid hormone ingestion, McCune-Albright syndrome, struma ovarii, and TSH-producing pituitary adenomas. Epidemic hyperthyroidism has also been seen when thyroid tissue has been inadvertently included in meat products.151 In contrast to these disorders, thyroid hormone resistance may appear similar to hyperthyroidism yet is best left untreated.
Graves disease
Graves disease is the most common cause of hyperthyroidism in children and adults and occurs when the thyroid gland is stimulated by immunoglobulins.51,148,152 In children, the incidence of Graves disease is about 1:10,000.153 Current treatment approaches for Graves disease include the antithyroid drugs (ATDs) propylthiouracil (PTU) or methimazole (MMI), surgery, and radioactive iodine (RAI; 131I), therapies that have been used since the 1960s.148,154–157
Medical therapy
PTU and MMI reduce thyroid hormone synthesis by inhibiting the oxidation and organic binding of thyroid iodide.158 Importantly, these medications are not curative. Rather, they palliate the hyperthyroid state until it spontaneously resolves or definitive treatment is rendered. In 2008, serious complications related to the use of PTU in children were noted and a review of adverse events related to ATD use in the pediatric population was reported.153 The risk of PTU-induced liver failure leading to transplantation was estimated to be 1 in 2000 children. The number of children developing PTU-induced liver injury that was reversible was estimated to be at least 10-fold greater than the number of children who develop liver failure requiring transplantation. Because PTU-induced liver injury is of rapid onset and can be rapidly progressive, biochemical monitoring of liver function tests and transaminase levels is not useful in managing the hepatotoxicity risk in a PTU-treated patient.153 Considering the risk of PTU-related hepatotoxicity in children, it is now recommended that the use of PTU be stopped, and children taking the medication should be considered for alternative treatments.159
Although PTU use should be avoided in favor of MMI, there is a role for the limited use of PTU. PTU use should be considered in circumstances when neither prompt surgery nor 131I treatments are readily available options, or when a toxic reaction to MMI has occurred but therapy for Graves disease is necessary. In this situation, PTU use should be used for the short term only. Because of potential teratogenic effects of MMI,160 PTU also is the drug of choice over the first trimester of pregnancy.161 In the United States and many other countries, MMI (or carbimazole) is available and is the drug of choice for Graves disease. The typical MMI dose is 0.2 to 0.5 mg/kg/day, with a range from 0.1 to 1 mg/kg/day.152 Although many practitioners give MMI in divided administered doses, data do not support a need for such titration.162
Overall MMI has a better safety profile than PTU, but MMI can be associated with minor adverse events;163 major adverse events include agranulocytosis and allergic reactions. Agranulocytosis has been reported in about 0.3% of adult patients taking MMI or PTU.156,164,165 Agranulocytosis is dose dependent with MMI and rarely occurs at low doses.156,164,165 When it develops, agranulocytosis occurs over the first 100 days of therapy in 95% of individuals.156,164,165 If patients taking MMI develop fever, pharyngitis, or feel ill, the medication should be immediately discontinued by the patient, a physician contacted, and a white blood cell count obtained.
The issue of how long anti-thyroid drugs (ATDs) should be used in children before considering radioactive iodine or surgery is a topic of controversy and warrants further study. Prospective studies in adults show that if remission does not occur within 18 months, there is little chance of remission with prolonged therapy.166 In children, when ATDs are used for 1 to 2 years, remission rates are generally 20% to 30%.167–169 The chance of remission after 2 years of ATD use will be low if the thyroid gland is large (> 2.5 normal size for age),170 the child is young (< 12 years)168,169,171 not Caucasian, initial serum TRAb levels are high, or FT4 levels are high at diagnosis (> 4 ng/dL; 50 pmol/l). 169
Studies of large cohorts of pediatric patients with Graves disease treated with ATDs for extended periods170,172 have revealed low remission rates that are comparable to those seen with 2 years of therapy. In view of these data, many practitioners will consider a trial of MMI for 1 or 2 years and proceed to surgery or 131I therapy if remission does not occur. Practitioners may also elect to continue ATDs for many years, as long as toxic reactions and progressive thyromegaly do not occur. Most recently, higher remission rates for children treat with antithyroid medications have been reported.173 Yet, these studies are at variance with most other studies148 and warrant additional follow-up.
Radioactive iodine therapy
The use of RAI has been reported in more than 1200 children.157 Patients as young as 1 year of age have been treated with 131I with excellent outcomes.157 Overall studies of 131I use in children report remission rates that exceed 95%.157,174,175 The goal of 131I therapy for Graves disease is to induce hypothyroidism. 131I doses are typically calculated to deliver the desired amount of radiation based on gland size and 24-hour 123I uptake. Some centers administer all patients the same fixed dose of 131I with excellent outcome.176 To achieve thyroid ablation or hypothyroidism, more than 150 uCi of 131I per gram of thyroid tissue should be administered.177,178 With larger glands (30 to 80 g), higher administered doses of 131I (200 to 300 uCi of 131I per g) may be needed.177 To assess gland size when the thyroid is large, ultrasonography is recommended with gland size determined by the formula, lobe size = [length × width × depth × 0.6] and the volumes of each lobe summed. Radioactive iodine is often not effective with large glands (> 80 g).179 Thus, surgery may be preferable to 131I in these patients, although patients can be given a repeat RAI treatment.
Some centers administer a fixed dose of about 15 mCi 131I to all children176 rather than providing individually calculated doses. One potential advantage of calculated versus fixed dosing, though, is that it may be possible to administer lower doses of 131I when the administered dose is calculated, especially when uptake is high. Less than 10% of children complain of mild tenderness over the thyroid in the first week after therapy, which can be treated effectively with acetaminophen or nonsteroidal anti-inflammatory agents for 24 to 48 hours175,177 There are rare reports of pediatric patients with severe hyperthyroidism who have developed thyroid storm after receiving 131I.180 Thyroid storm in this setting is believed to reflect progression of the uncontrolled hyperthyroid state. Thus, if T4 values are > 20 μg/dL or freeT4 values are > 5 ng/dL (60 pmol/l), children should be treated with MMI until T4 or free T4 levels normalize before proceeding with 131I therapy.177
Hypothyroidism typically develops by 2 to 3 months posttreatment.176,177 When administered doses > 150 uCi of 131I per gram of thyroid tissue are administered, hypothyroidism rates are about 95%.152,157,181 If hyperthyroidism persists 4 to 6 months after therapy, retreatment with 131I is indicated.
The thyroid gland is unique in its developmental sensitivity to malignancy following low-level radiation exposure.182,183 When individuals are younger than 20 years at the time of exposure to low-level thyroid irradiation, thyroid cancer risks increase the younger the patient is at the time of exposure.182,183
Detractors of 131I therapy point to the increased rates of thyroid cancer and thyroid nodules observed in young children exposed to radiation from nuclear fallout at Hiroshima or after the explosion at the Chernobyl nuclear reactor.183,184
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree