Overview and principles of pediatric endocrinology
Historical background
Endocrinology is a discipline of science that seeks to understand how chemical signals secreted by cells regulate the function of distant (endocrine) or local (paracrine) tissues, or even their own function (autocrine), in order to integrate vital processes of life such as growth, reproduction, and metabolism (Figure 1-1). Classical endocrinology derived from careful clinical observation such as, for example, the gigantism associated with pituitary tumors or the characteristic bodily changes now known as Cushing disease which is also associated with pituitary tumors; histology indicated the former was likely the result of a product made by “acidophilic cells,” whereas the latter was associated with the expansion of “basophilic cells.” Unlike the chemical substances secreted into ducts leading to a target tissue (“exocrine”), the products of these acidophilic or basophilic cells had to traverse the bloodstream in order to reach their distant and often multiple targets. Hence, these were internal (“endocrine”) secretions. Cushing disease was associated with hypertrophy of the adrenal cortex and certain tumors of these tissues mimicked the features of Cushing disease.1 Hence, it was readily postulated that the pituitary secretes a substance that affects the adrenal glands and function; this substance was named adrenocorticotrophic hormone (ACTH) and it was deduced that the features of Cushing disease/syndrome were the result of a product or products from the adrenal gland. The destruction of adrenal tissue by tuberculosis or tumor was identified by Thomas Addison in 1855 and treatment of this entity with adrenal extracts, resulting in marked improvement, was first undertaken by William Osler in 1896.2 However, the purification of these “internal secretions” began in earnest only at the turn of the 20th century with spectacular success as reviewed by Dr. Delbert Fisher.2 In the first quarter of the 20th century, epinephrine, thyroxin, insulin, and parathyroid hormone (PTH) were purified, followed by the purification of the sex steroids from the ovary and testes as well as the pituitary and placental gonadotropins that stimulated the gonads to secrete these substances. These purifications required laborious chemical methods and the elucidation or measurement of their function required costly and cumbersome bioassays. For example, the assay of insulin potency, discovered in 1921, required the use of rabbits; the definition of 1 international unit (IU) of insulin was assigned to be the amount of insulin that lowers the blood glucose of a healthy 2-kg rabbit, fasted for 24 hours, to 45 mg/dL within 5 hours of injection. Clearly, such potency estimates reflected the relative crudeness of the purification; today’s recombinant human insulin possesses approximately 29 IU/mg, whereas the potency of porcine insulin in the early 1980s was ∼23 IU/mg and likely less at the dawn of insulin therapy for diabetes. Moreover, the lack of sensitivity in such assays prohibited the ability to measure this substance in normal blood or other biologic fluids; refinements such as measuring the incorporation of labeled glucose into the fat pad or diaphragm of a rat represented only an incremental improvement.3 Growth hormone (GH), isolated in 1944, was assayed by its ability to increase the width of the tibia growth plate in rats after a defined period of injections and by comparing the unknown relative to a dose response of known concentrations administered in vivo.4 Attempts to improve sensitivity and specificity led to the “sulfation factor-somatomedin hypothesis” (Figure 1-2), in which it was postulated that GH leads to the generation of a second substance, derived from the liver, which mediates the growth-promoting (somatotropic) effects and hence was named “somatomedin.”4,5 Subsequent studies demonstrated that this substance was identical to a factor in serum, which had insulin-like properties in vitro, that was retained even after all insulin was “quenched” by an excess of antibodies specific for insulin. The convergence of these two pathways eventually led to the discovery of the factor now known as insulin-like growth factor (IGF)-I.5 Despite these limitations, the scientific curiosity of these chemical substances that regulated functions as diverse as blood pressure (epinephrine, cortisol), water metabolism (arginine vasopressin [AVP], cortisol), growth (GH), glucose (insulin, cortisol), and reproduction (sex steroids, follicle-stimulating hormone [FSH], luteinizing hormone [LH]) spurred the formation of medical societies focused on endocrine diseases. As detailed in the article by Fisher from which the following historical aspects are quoted,2 the Association for the Study of Internal Secretions was established in 1918 in the United States and renamed the Endocrine Society in 1952.
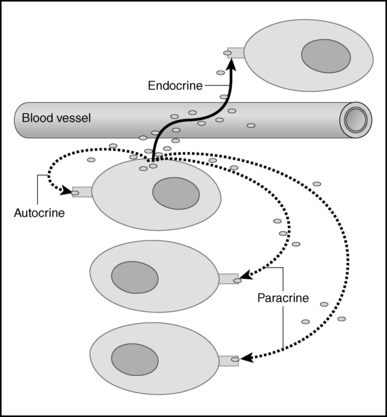
FIGURE 1-1  Cellular signaling. Chemical signals synthesized and secreted by cells, may be released into the bloodstream to be distributed to target cells with specific capability to respond to the signal. These blood-borne chemicals constitute classical endocrine signals, also known as “internal secretions,” to distinguish them from the chemicals secreted into a duct that leads directly to another organ (e.g., pancreatic enzymes destined for the duodenum via the pancreatic duct [“exocrine”]). However, the same cell may release the chemical that then affects nearby cells without traversing the bloodstream (these are known as paracrine effects) or act on a receptor on its own surface to modify the cells own functions (autocrine). (From King TC [2006]. Elsevier’s integrated pathology. Philadelphia: Mosby, Figure 3-6.)
Cellular signaling. Chemical signals synthesized and secreted by cells, may be released into the bloodstream to be distributed to target cells with specific capability to respond to the signal. These blood-borne chemicals constitute classical endocrine signals, also known as “internal secretions,” to distinguish them from the chemicals secreted into a duct that leads directly to another organ (e.g., pancreatic enzymes destined for the duodenum via the pancreatic duct [“exocrine”]). However, the same cell may release the chemical that then affects nearby cells without traversing the bloodstream (these are known as paracrine effects) or act on a receptor on its own surface to modify the cells own functions (autocrine). (From King TC [2006]. Elsevier’s integrated pathology. Philadelphia: Mosby, Figure 3-6.)
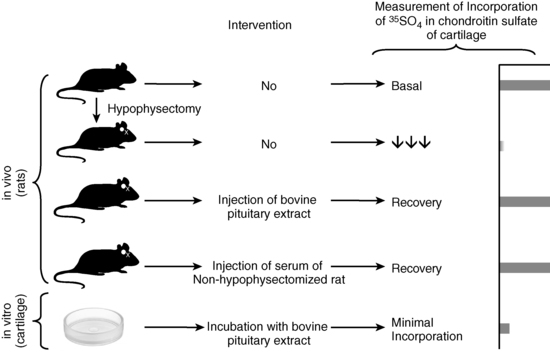
FIGURE 1-2  “Sulfation factor” assay of growth hormone. The bioassay of growth hormone (GH) consisted of administering graded doses of bovine GH with potency approximately 1.25U/mg via daily subcutaneous (SC) injections for 4 days to young, growing, prepubertal rats (approximately 31 days) that had been hypophysectomized 10 days previously. Approximately 5 animals per group and about 5 doses (0 plus 4 graded increments) were used to construct the “dose-response curve” of the increase in the width of the tibia growth plate; the unknown was 2 to 3 mL of plasma also administered SC to approximately three to five animals per test dose. The sulfation assay was an attempt to refine the technique by examining the dose-response relationship between the incorporation of 35SO4 into chondroitin sulfate in vivo, or in vitro into uniformly prepared cartilage rings obtained from young rats. As shown in the figure, the in vivo component examined basal activity of the animal’s serum on the amount of radioactivity incorporated in vivo. Hypophysectomy almost completely abolished this ability to stimulate 35SO4 incorporation, but injection of bovine pituitary extract or serum from a nonhypophysectomized animal restored this activity. However, in vitro incubation of cartilage rings with bovine pituitary extract resulted in only minimal incorporation. Thus, it was proposed that GH acted on an internal organ to produce the “sulfation factor.” The sensitivity of these assays were, at best, in the 1 to 10 µg/mL range, and precision and reproducibility were poor (Tweed DC, McCullagh EP [1962]. Assay of growth hormone-like activity in blood plasma: a comparison of two methods. Clin Chem 8:141-150; also see references 4 and 5). Today’s immunoassays permit measurement of plasma concentrations of GH with 1000-fold greater sensitivity than these early bioassays (ng/mL compared to μg/mL above) with high degrees of precision and reproducibility (Chapter 4). (The author is indebted to Oscar Escobar, MD, Associate Professor, Department of Pediatrics, University of Pittsburgh School of Medicine, Division of Pediatric Endocrinology, Children’s Hospital of Pittsburgh, for creating this figure and granting permission for its use in this chapter.)
“Sulfation factor” assay of growth hormone. The bioassay of growth hormone (GH) consisted of administering graded doses of bovine GH with potency approximately 1.25U/mg via daily subcutaneous (SC) injections for 4 days to young, growing, prepubertal rats (approximately 31 days) that had been hypophysectomized 10 days previously. Approximately 5 animals per group and about 5 doses (0 plus 4 graded increments) were used to construct the “dose-response curve” of the increase in the width of the tibia growth plate; the unknown was 2 to 3 mL of plasma also administered SC to approximately three to five animals per test dose. The sulfation assay was an attempt to refine the technique by examining the dose-response relationship between the incorporation of 35SO4 into chondroitin sulfate in vivo, or in vitro into uniformly prepared cartilage rings obtained from young rats. As shown in the figure, the in vivo component examined basal activity of the animal’s serum on the amount of radioactivity incorporated in vivo. Hypophysectomy almost completely abolished this ability to stimulate 35SO4 incorporation, but injection of bovine pituitary extract or serum from a nonhypophysectomized animal restored this activity. However, in vitro incubation of cartilage rings with bovine pituitary extract resulted in only minimal incorporation. Thus, it was proposed that GH acted on an internal organ to produce the “sulfation factor.” The sensitivity of these assays were, at best, in the 1 to 10 µg/mL range, and precision and reproducibility were poor (Tweed DC, McCullagh EP [1962]. Assay of growth hormone-like activity in blood plasma: a comparison of two methods. Clin Chem 8:141-150; also see references 4 and 5). Today’s immunoassays permit measurement of plasma concentrations of GH with 1000-fold greater sensitivity than these early bioassays (ng/mL compared to μg/mL above) with high degrees of precision and reproducibility (Chapter 4). (The author is indebted to Oscar Escobar, MD, Associate Professor, Department of Pediatrics, University of Pittsburgh School of Medicine, Division of Pediatric Endocrinology, Children’s Hospital of Pittsburgh, for creating this figure and granting permission for its use in this chapter.)
Pediatric endocrinology began as a subspecialty only in the 1940s with the establishment of endocrine clinics at the Massachusetts General Hospital and Johns Hopkins. These programs attracted postdoctoral trainees who then established their own pediatric endocrine units in the burgeoning growth of academic medical centers in the 1950s and 1960s. In the United States, the Pediatric Endocrine Society, first named the Lawson Wilkins Pediatric Endocrine Society (LWPES), was formed in 1972 and established as a subspecialty by the American Board of Pediatrics with its first certification examination in 1978; there are more than 1000 board-certified pediatric endocrinologists today in the United States. The European Society for Pediatric Endocrinology was formed in 1966, followed by the Japanese Society for Pediatric Endocrinology in 1967 and the British Pediatric Endocrine Group in 1972, all preceding the LWPES in the United States. Several other regional pediatric endocrine groups were formed, including the Australian Pediatric Endocrine Group, the Sociedad Latino Americana de Endocrinologia Pediatrica, and the Asia Pacific Pediatric Endocrine Society. All of these groups now meet jointly every 4 years at an International Pediatric Endocrine Congress.2
Impact of hormonal assays and molecular biology
Two discoveries revolutionized the field of endocrinology and led to an explosion of basic, clinically relevant therapeutic knowledge in the second half of the 20th century. The first was the development of radioimmunoassay by Yalow and Berson, reported for insulin in 1960.6 Here was a method for measuring the low concentrations of a hormone using as little as 10 to 50 μL of a biological fluid in an accurate, reproducible way with precision and sensitivity adequate for in vivo studies in humans or other species, as well as in vitro studies, such as the regulation of insulin secretion by nutrients, hormones, ions, and pharmaceutical agents in whole animals including humans, in vivo, or in isolated perfused pancreas, or in isolated islets. This was followed by the rapid development of assays for various hormones and an explosion of discovery, including the distinction between absolute and relative insulin deficiency as the difference between “juvenile” and “maturity onset” diabetes, the regulation of GH secretion in normal individuals at different ages and in clinical disorders of growth, the changes in thyroid function at birth and the possibility of screening for neonatal hypothyroidism, and the changes in gonadotropins and sex hormones during the process of normal and abnormal puberty. The discovery and purification of the hypothalamic releasing hormones for TSH, FSH/LH, GH, and ACTH were made possible by these precise assays using (rat) pituitary cells perfused by protein fractions derived from the hypothalami of animals.7 The discovery that a hormone produced in a cell could affect the function of its neighboring cell(s), without traveling through the bloodstream (paracrine action) or even its own function (autocrine), was also enabled by the use of these sensitive and precise tools, expanding our concepts of a hormone as a chemical messenger that influences, directs, and coordinates cellular functions throughout the body (see Figure 1-1). Similar principles enabled the identification of the receptor molecules at the cell surface or in its cytoplasm that permit the hormone signal to be transduced to a message for turning biological processes on, or off, in specific tissues.8 Refinements using the principles of radioimmunoassay (RIA) but without radioactivity are the bases of modern laboratory methodologies for hormone measurement as well as for other chemical substances such as drugs; examples of modern application of these methods as well as the pitfalls are reviewed in Chapter 4. Signal transduction pathways and their relevance to pediatric endocrinology are discussed in Chapter 3. The notion that a hormone may not be capable of eliciting a response despite high concentrations was implicit in the entity labeled “pseudohypoparathyroidism” by Dr. Fuller Albright9
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree