Autoimmune polyglandular syndromes
Introduction
The autoimmune polyglandular syndromes (APS I and II) are uncommon constellations of organ-specific autoimmune diseases characterized by the occurrence of more than one autoimmune disease in an affected individual (Table 20-1). More commonly, autoimmune disease of endocrine glands occurs in only a single organ, but multiorgan involvement of both endocrine and nonendocrine organs and tissues may be present.
TABLE 20-1
The Autoimmune Polyglandular Syndromes I and II
| APS I | APS II | |
| Comparative frequency Onset Heredity Gender Genetics Hypoparathyroidism Mucocutaneous candidiasis Ectodermal dysplasia Addison disease Type 1 diabetes Autoimmune thyroid disease Pernicious anemia Gonadal failure Females Males Vitiligo Alopecia Autoimmune hepatitis Malabsorption | Less common Infancy/early childhood Autosomal recessive Males = females AIRE gene; no HLA association 77%-89% 73%-100% 77% 60%-86% 4%-18% 8%-40% 12%-15% 30%-60% 7%-17% 4%-13% 27% 10%-15% 10%-18% | More common Late childhood, adulthood Polygenic Female predominance HLA associated; DR/DQ None None None 70%-100% 41%-52% 70% 2%-25% 3.5%-10% 5% 4%-5% 2% Rare Rare |
Tolerance is an active state in which the immune system does not mount a reaction against self-antigens.1–4 If tolerance is not established or is lost, autoimmunity and subsequent disease may result. Although the breakdown in self-tolerance remains mostly unexplained, our improved understanding of the complex interplay between genetics and environment and the resultant aberrant immunologic processes has identified a number of possible mechanisms.5 To comprehend these mechanisms, a brief overview of how tolerance is maintained is essential.
Mechanisms underlying generation of autoimmunity
Introduction
In a normal immune response, the host organism must differentiate self from nonself, initiate an immune response to nonself, and eliminate nonself to protect the host from injury, organ dysfunction, and even death.1–4 Endogenous antigens represent self, whereas exogenous antigens represent nonself. The adaptive immune system assumes all exogenous antigens are potentially harmful and acts to eliminate nonself. Self-nonself discrimination is carried out by the adaptive (specific) immune system through the use of T- and B-cell surface receptors.6–8 These receptors recognize distinctive peptides (e.g., the T-cell receptors) or epitopes (e.g., the B-cell antibody receptors) and are the keys to the specificity of the adaptive immune response. Whereas B cells and their receptors recognize soluble antigen or antigens on cell surfaces, T cells and their receptors only perceive short polypeptides presented by specialized cell-surface molecules encoded by the major histocompatibility complex (MHC).9–11 The human MHC is termed the human leukocyte antigen (HLA) complex. Class I MHC (e.g., HLA-A, HLA-B, and HLA-C molecules) present peptides predominantly derived from the cell cytoplasm to T cells. T cells are classified on the basis of their cluster of differentiation (CD) surface proteins, which bind differentially to antigens presented on class I and class II MHC. For example, CD8 cells, considered “cytotoxic” T cells, typically recognize targets presented on class I MHC. In addition, more “professional” antigen presenting cells known as dendritic cells and defined by a combination of CD markers and the characteristic presence of numerous membrane processes that extend out from the cell body can present peptides of extracellular origin via class I MHC molecules. Conversely, CD4 cells typically are activated when peptides derived from the extracellular space are presented via class II MHC (e.g., HLA-DP, HLA-DQ, and HLA-DR molecules).
Regulation of T-cell self-tolerance occurs at two distinct but interdependent levels: centrally and peripherally (described in detail later). Central tolerance occurs in the thymus via positive and negative selection of self-reactive T cells, whereas peripheral tolerance occurs in both lymphoid and nonlymphoid tissues (Figures 20-1 and 20-2). Although many of the mechanisms involved in establishing tolerance remain poorly understood, nearly 15 years of characterizing the autoimmune regulatory gene (AIRE) has improved understanding of positive and negative T-cell selection.4 The AIRE gene, which codes for a transcription factor found in the medulla of the thymus, plays a critical role in the ectopic expression of tissue-specific antigens, which regulate negative selection of autoreactive T effector cells.12,13 Deletions in the mouse AIRE homologue result in multiorgan autoimmunity, whereas mutations in the human AIRE gene result in APS I.14,15
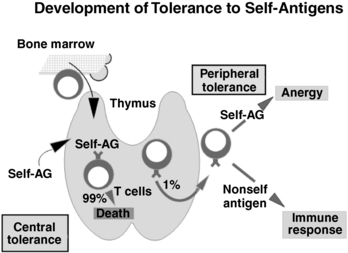
FIGURE 20-1  Normal tolerance pathways. T-cell precursors initially arise in the bone marrow. These progenitors enter the thymus, and developing T cells encounter self-antigens. Strong self-antigen stimulation of developing T cells induces apoptosis, with approximately 99% of all developing T cells dying. This is central immunologic tolerance where strongly antiself reactive T cells are eliminated. Naïve T cells that do leave the thymus can be subsequently tolerized to self-antigens if they encounter self-antigen without the normal costimulatory signals (B7.1/B7.2-CD28, see Figure 20-3). Induction of tolerance outside the thymus is termed peripheral tolerance (top right) and is a complementary mechanism to central tolerance. Peripheral tolerance is functionally expressed as anergy: autoreactive cells are present but are inactive (top right). If nonself-antigen is encountered, a normal immune response ensues (bottom right).
Normal tolerance pathways. T-cell precursors initially arise in the bone marrow. These progenitors enter the thymus, and developing T cells encounter self-antigens. Strong self-antigen stimulation of developing T cells induces apoptosis, with approximately 99% of all developing T cells dying. This is central immunologic tolerance where strongly antiself reactive T cells are eliminated. Naïve T cells that do leave the thymus can be subsequently tolerized to self-antigens if they encounter self-antigen without the normal costimulatory signals (B7.1/B7.2-CD28, see Figure 20-3). Induction of tolerance outside the thymus is termed peripheral tolerance (top right) and is a complementary mechanism to central tolerance. Peripheral tolerance is functionally expressed as anergy: autoreactive cells are present but are inactive (top right). If nonself-antigen is encountered, a normal immune response ensues (bottom right).
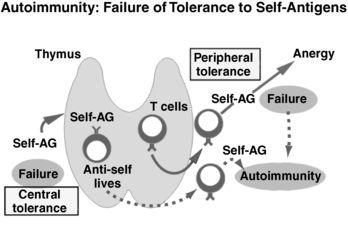
FIGURE 20-2  Autoimmunity: failure of tolerance to self-antigens. With a failure of central tolerance (bottom left), antiself T cells survive that should not normally survive. When these antiself T cells leave the thymus, they are able to produce autoimmunity (dotted arrows). Alternatively, with a failure of peripheral tolerance (top right), if anergy does not occur after contact with self-antigen, an autoimmune response can occur.
Autoimmunity: failure of tolerance to self-antigens. With a failure of central tolerance (bottom left), antiself T cells survive that should not normally survive. When these antiself T cells leave the thymus, they are able to produce autoimmunity (dotted arrows). Alternatively, with a failure of peripheral tolerance (top right), if anergy does not occur after contact with self-antigen, an autoimmune response can occur.
Central T-Cell tolerance
T cells are primarily educated to distinguish self and nonself in the thymus.16–19 Central T-cell tolerance is the process by which antiself T cells are eliminated in the thymus. As many as 99% of developing thymocytes die in the thymus and never reach the periphery.
T-cell tolerance is a function of the selection of the T-cell receptor (TCR) repertoire that exits the thymus. In the thymic cortex CD4+, CD8+ (double positive) T cells bearing alpha/beta TCRs that bind to self-MHC initially survive (positive selection). In this way the thymus initially chooses for survival T cells that bind to self-MHC as opposed to TCRs that might bind to other self molecules leading to noneffective communication. This positive selection resulting from self peptide-MHC presentation is carried out by thymic nurse epithelial cells in the cortex. At the corticomedullary junction of the thymus, if such saved TCRs bind to self-MHC too tightly, autoreactivity is possible and these T cells then undergo negative selection and suffer apoptotic death. The cells inducing negative selection are macrophages and dendritic cells. This process of positive selection for MHC binding in the thymic cortex and negative selection (at the thymic corticomedullary border) against tight binding to self-peptides accounts for central (thymic) immunologic tolerance.3,5,13,20
Peripheral T-Cell tolerance
Once in the circulation and secondary lymphoid organs (e.g., lymph nodes and spleen), naïve T cells still require multiple signals to become activated.7 The initial signal is the presentation of antigen-specific peptides to T-cell receptors by MHC molecules. CD4 and CD8 molecules on these T-cell subsets serve as antigen-nonspecific coreceptors binding to nonpolymorphic portions of the class II MHC molecules and class I MHC molecules, respectively. The second signal is antigen nonspecific and is provided by the B7.1 (CD80) and B7.2 (CD86) molecules of the antigen-presenting cell interacting with the CD28 molecule on the T-cell surface.
When the T cell perceives both signals, a cascade of intracellular signaling events occur, leading to T-cell activation. Activated CD4 T cells express numerous cytokines, cytokine receptors, and CTLA-4. Activated CD4 T cells then down-regulate T-cell receptor expression and acquire class II MHC expression. It is unknown why activated human T cells express class II MHC (activated T cells in mice do not express class II MHC). CTLA-4 expression by the activated T cell and its interaction with B7.1/B7.2 provides an immunosuppressive signal to the T cell, thereby down-regulating the T-cell immune responses. Thus, CTLA-4 and CD28 act antithetically: B7.1/B7.2-CD28 turns on T cells, whereas B7.1/B7.2-CTLA-4 down-regulates the T cell (Figure 20-3).
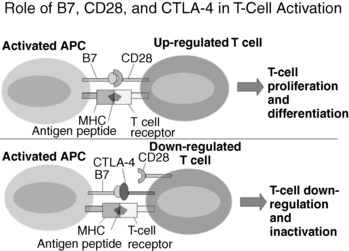
FIGURE 20-3  Role of B7, CD28, and CTLA-4 in T-cell activation. Activated antigen presenting cells (APC) present antigen peptides on the major histocompatibility complex (MHC) molecules and express B7, including B7.1 (CD80) and B7.2 (CD86), costimulators. When B7.1 or B7.2 is bound by CD28 and MHC plus peptide is bound by a T-cell receptor, T-cell proliferation and differentiation of naïve T cells ensues. Conversely, activated T cells express CTLA-4 and bind to B7 (either B7.1 or B7.2] inducing down-regulation and inactivation of T cells.
Role of B7, CD28, and CTLA-4 in T-cell activation. Activated antigen presenting cells (APC) present antigen peptides on the major histocompatibility complex (MHC) molecules and express B7, including B7.1 (CD80) and B7.2 (CD86), costimulators. When B7.1 or B7.2 is bound by CD28 and MHC plus peptide is bound by a T-cell receptor, T-cell proliferation and differentiation of naïve T cells ensues. Conversely, activated T cells express CTLA-4 and bind to B7 (either B7.1 or B7.2] inducing down-regulation and inactivation of T cells.
Helper CD4 T cells have classically been subdivided into two distinct lineages: (1) Th1 cells, which activate cell-mediated and some antibody responses, and (2) Th2 cells, which predominantly activate antibody-mediated responses.21 However, additional T-cell lineages exist (i.e., Th17 cells, T-follicular helper cells, and regulatory T cells) and data have described remarkable plasticity in their cytokine expression, suggesting shifting T-cell functionalities depending on environmental cues.22 Although overly simplistic, Th1 subsets can be thought of as cells that activate macrophages, natural killer cells, and B cells and secrete predominantly IL-2, interferon gamma (IFN-γ), tumor necrosis factor–beta (TNF-β), and IL-12. Th2 cells elaborate IL-4, IL-5, IL-6, IL-10, and IL-13. Crosstalk between Th1 and Th2 cells occurs; for example, IFN-γ from Th1 cells suppresses Th2 cells and IL-10 from Th2 cells inhibits Th1 cells. Another subset of CD4 T cells has been described as regulatory T cells that secrete the immunomodulatory cytokines IL-10 or transforming growth factor–beta (TGF-β). Regulatory T cells include CD4+CD25+FoxP3+ T cells, Tr1, and Th3 cells. Tr1 and Th3 cells express CD4 but do not express CD25 (the IL-2 receptor alpha chain). Tr1 cells secrete IL-10 and TGF-β, whereas Th3 cells secrete IL-4, IL-10, and TGF-β. Although CD4+CD25+FoxP3+ T cells can secrete both IL-10 and TGF-β, their regulatory action on autoreactive T cells appears to occur through cell-to-cell contact. Upon activation, CD8 T cells, often with the help of Th1 cells supplying IFN-γ to up-regulate B7 expression on antigen presenting cells, become functional cytotoxic T killer cells.
Ongoing characterization of regulatory T cells has improved our understanding of peripheral tolerance. Regulatory T cells play a critical role in suppressing the activity of effector T cells that escape negative selection to self-antigen in the thymus.23 Functional regulatory T cells are able to anergize previously self-reactive T effector cells, resulting in improved tolerance to self. The expression of the forkhead transcription factor, FoxP3, is specific for identification of the CD4+CD25+ cell (Treg) population. First identified in the Scurfy mouse, a mouse model of immune dysfunction and polyendocrinopathy, abnormal FoxP3 expression is now known to be responsible for the failure in tolerance in humans affected with a similar polyendocrinopathy discussed later.24–26 The absence of normal FoxP3 expression in humans leads to an extremely rare, X-linked, recessively inherited, and typically fatal autoimmune lymphoproliferative disease known as immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance (IPEX).27,28 Defects in the forkhead transcription factor FoxP3 that are responsible for IPEX map to Xp11.23-Xq13.3.
B-cell tolerance
B cells are partially educated in the bone marrow to be anergized or undergo apoptosis in response to self-antigen when they are at the stage of development of the naïve immature B cell (central B-cell tolerance). In the bone marrow, naïve immature B cells that see multivalent antigens become anergized (unresponsiveness to subsequent stimulation), whereas exposure to highly polyvalent antigens can induce apoptosis.29
Autoimmune diseases
The organ-specific nature of many autoimmune diseases results from abnormal immune system recognition of tissue-specific self-antigens. In many autoimmune endocrinopathies, the target molecule is either a tissue-specific or tissue-limited (i.e., the protein is not unique to one tissue but is clearly restricted in its distribution) enzyme or cell-surface receptor30,31 (Table 20-2).
TABLE 20-2
Autoantigens in Autoimmune Endocrine and Associated Diseases
| Disease | Autoantigens | Putative Autoantigens |
| Mucocutaneous candidiasis | IL-17A IL-17F IL-22 | |
| Hypoparathyroidism | NALP5 (specific for APS) CaSR | |
| Addison disease | P450c21 | P450c17 P450scc |
| Hashimoto thyroiditis | Thyroperoxidase Thyroglobulin | |
| Graves disease | Thyrotropin receptor | |
| Diabetes | Insulin Glutamic acid decarboxylase65 IA-2 (ICA 512) IA-2β ZnT8 | Proinsulin Carboxypeptidase H ICA69 Glima 38 |
| Premature gonadal failure | P450scc | P450c17 3β hydroxysteroid dehydrogenase |
| Pernicious anemia | H+/K+ ATPase pump Intrinsic factor | |
| Myasthenia gravis | Acetylcholine receptor α chain | |
| Vitiligo | Tyrosinase Tyrosinase related protein-2 L-amino acid decarboxylase | |
| Celiac disease | Endomysium transglutaminase | Reticulin Deamidate gliadin |
| Autoimmune hepatitis | Liver kidney microsome 1 | L-amino acid decarboxylase Tryptophan hydroxylase |
The criteria for classification of a disease as autoimmune are not universally agreed upon.32 However, major criteria that are generally accepted as strong evidence that the disease is autoimmune include (1) detection of autoantibodies or autoreactive T cells including lymphocytic infiltration of the targeted tissue or organ, (2) disease transfer with antibodies or lymphocytes, (3) disease recurrence in transplanted tissue, and (4) ability to abrogate the disease process with immunosuppression or immunomodulation. Few, if any, human autoimmune diseases meet all four of these criteria. Further information that is supportive of, but not diagnostic for, an autoimmune disease includes (1) increased disease frequency in women compared to men, (2) the presence of other organ-specific autoimmune diseases in affected individuals, and (3) increased frequencies of particular HLA alleles in affected individuals.
Defects in tolerance that cause autoimmune diseases
Several hypotheses explaining defects in tolerance have been proposed.33 Theoretically autoimmunity may develop because (1) tolerance never developed to specific self-antigens or (2) established tolerance was lost. If self-antigen is not efficiently presented in the thymus, tolerance may not be established during T-cell education within the thymic cortex.34 For example, variations in the insulin gene VNTR (variable number of tandem repeats), ∼500 base pairs upstream of the insulin gene promoter, influence the extent of insulin gene expression in the thymic cortex. The risk of developing type 1 diabetes is enhanced when certain VNTR alleles are present, which leads to lower mRNA expression of insulin in the thymus. Specifically, certain protective class III alleles are associated with increased thymic expression of insulin and a decreased risk of developing type 1 diabetes, whereas class I alleles are associated with decreased thymic expression of insulin and an increased risk of developing diabetes.35 Failure to delete specific autoreactive T cells clones predisposes patients to autoimmunity. If autoimmunity does result from defects in thymic tolerance, the defects must be antigen specific because organ-specific autoimmune diseases are usually extremely selective. For example, in type 1 diabetes, whereas the beta cells are attacked and ultimately destroyed by a cell-mediated autoimmune process, the remaining islet cells including alpha cells, delta cells, and pancreatic polypeptide-producing cells are unscathed.
Defects in peripheral tolerance could result from concurrent T-cell stimulation by self-antigen/MHC plus T-cell costimulation (e.g., B7.1/B7.2-CD28) leading to aberrant T-cell activation and an autoimmune response. If tolerance has not been developed because an antigen is sequestered intracellularly or is not expressed in the thymus during T-cell ontogeny, T-cell reactivity in the periphery would not be abrogated. However, several antigens initially thought to be sequestered intracellularly have now been shown to circulate in low concentrations in normal individuals. Thyroglobulin is such a self-antigen in autoimmune thyroid disease. The development of thyroglobulin autoantibodies was believed to follow the release of thyroglobulin from the thyroid gland following viral infection or trauma. “Immunization” with thyroglobulin would hypothetically lead to an antithyroglobulin humoral response and autoimmune thyroid disease would follow. However, we now know that thyroglobulin does circulate in low but appreciable quantities in normal individuals who show no serologic evidence of thyroid autoimmunity. Furthermore, thyroid follicular cell destruction in Hashimoto thyroiditis is cell mediated and not humorally mediated.
If sequestered antigens do play a role in autoimmune disease, viral infections, trauma, ischemia, or irradiation are all mechanisms that could disturb cellular integrity and lead to the release of intracellular antigens.36 Some self-antigens may never normally come into contact with the immune system unless there is a breakdown of anatomic barriers within the body. An example is the occurrence of autoimmunity to the eye following orbital trauma. Although a rare consequence of orbital damage, initiation of an autoimmune response to eye proteins in adjacent lymph nodes can generate autoreactive T cells that can invade and damage the contralateral eye (“sympathetic ophthalmia”).37,38 Removal of the inciting damaged tissues and immunosuppression may be required to sustain vision in the undamaged eye. Similarly, transient autoantibody reactivity to cardiac myosin following myocardial infarction has also been described.39
Alteration of self-antigens as a result of infection or neoplasia is believed to be a plausible theory explaining some types of autoimmunity. As environmental triggers, viral infections could lead to modification of self-proteins and neoantigen expression (e.g., a new antigen is present on self-cells). Alternatively, a self-antigen may be partially degraded leading to a “new” antigenic target for the adaptive immune system. This new antigen is recognized as foreign by the immune system and the immune response to these new antigens results in autoimmunity.
Molecular mimicry is one of the most popular explanations for autoimmunity.36,40 Due to exposure to a dietary, viral, or bacterial antigen (e.g., infection) and molecular mimicry (similarity) between the self-antigen and the foreign antigen, the immune response to the foreign antigen leads to cross reactivity with self-antigen, autoimmunity, and disease.40–43 For this theory to work, tolerance must not previously exist to the self-antigen. This might be true if the self-antigen is truly sequestered and the immune system has never developed tolerance to the self-antigen. Alternatively, the self-antigen peptides may be present in too low a concentration to elicit an immune response and initial immune system tolerization has not occurred. Only with infection or novel dietary exposure would there be a sufficient degree of self-immunization to develop immune autoreactivity. With immune autoreactivity, self is now recognized as foreign during the response to the cross-reactive pathogen. If the self-antigen is a cell surface antigen, the “pathogen-induced” autoantibodies could fix to self and produce disease via complement fixation, or the antibodies could act as opsonins for fixed or circulating phagocytes (antibody-dependent-cell cytotoxicity). In rheumatic fever, cross-reactivity between Streptococcus M protein and cardiac myosin have been described. In ankylosing spondylitis, cross-reactions between Klebsiella nitrogenase and HLA-B27 have been described. In rheumatoid arthritis there is cross-reactivity between cartilage protein and a mycobacterial proteoglycan wall component.
Some cases of autoimmunity may result from superantigens initiating an antiself immune response as part of the polyclonal immune activation process. Superantigens are polyclonal T-cell stimulators that have the ability to cross-link TCR beta chains and MHC molecules. Superantigens have been reported to activate as many as one third of all T cells in the body. In such cases, systemic disease can develop from massive cytokine release (e.g., the systemic inflammatory response syndrome [SIRS]). This is the case in toxic shock syndrome, wherein a staphylococcal exotoxin acts as a superantigen. Mycobacterial antigens have also been proposed as possible superantigens in Crohn disease.44 This theory presupposes that T cells bearing antiself TCRs have not been deleted or permanently anergized. T cells with antiself receptors may be stimulated, and if they encounter self-antigen, they may further proliferate to develop an autoimmune response.
However, which of these speculative theories applies to the APS is unknown. Human disease most often results from an interaction of environmental and genetic factors.45–47 Many environmental factors are implicated in various autoimmune diseases: wheat gliadin ingestion and celiac disease, penicillamine exposure and myasthenia gravis, methimazole and autoimmune hypoglycemia from insulin autoantibodies (seen primarily in Japanese patients), and amiodarone and thyroiditis. Even cancer can be associated with the development of autoimmunity: thymoma and myasthenia gravis,48 ovarian teratoma and N-methyl-D-aspartate (NDMA) receptor meditated encephalitis,49 and breast cancer and stiff person syndrome.50 Despite remarkable improvements in our understanding of immunology, many achieved through meticulous studies of APS, the mechanisms whereby the complex interaction of genes, environment, and immune system lead to autoimmunity remain to be fully elucidated.
Classification of the autoimmune polyglandular syndromes
APS I, also known as autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED), is an autosomal recessive disorder mapped to a single gene (the autoimmune regulator or AIRE gene) on chromosome 21q22.3.14,15 The presence of two of the following three conditions are prerequisites for the diagnosis of APS I: (1) adrenocortical failure (Addison disease) or serologic evidence of adrenalitis (adrenal autoantibodies), (2) hypoparathyroidism, and (3) chronic mucocutaneous candidiasis.45,46,51–54 APS II is defined by the coexistence of autoimmune adrenocortical insufficiency or serologic evidence of adrenalitis with autoimmune thyroiditis (Schmidt syndrome) or type 1 diabetes mellitus (Carpenter syndrome: Schmidt syndrome plus type 1 diabetes) or serologic evidence of either thyroid or islet autoimmunity45,55–58 (Figure 20-4). The presence of thyroiditis without adrenal disease but associated with either type 1 diabetes, pernicious anemia, vitiligo, or alopecia has been referred to by some authors as APS III, whereas additional combinations of autoimmune disease have been referred to as APS IV (i.e., vitiligo plus alopecia, type 1 diabetes plus celiac disease, or type 1 diabetes and vitiligo).59 However, because APS III and IV differ from APS II only by the presence or absence of adrenocortical disease (and share similar susceptibility genes and immunologic features), we do not recognize APS III or IV as unique syndromes and consider them extensions of the APS II constellation.
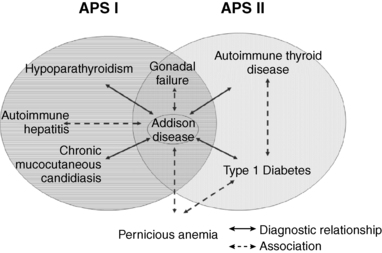
FIGURE 20-4  Diagnostic relationships and common associations in APS I and APS II. The solid lines indicate diagnostic relationships. The dashed lines indicate common associations. The diagnosis of APS I depends on the coexistence of Addison disease (or adrenal autoantibodies) plus either hypoparathyroidism or chronic mucocutaneous candidiasis or both. The diagnosis of APS II depends on the coexistence of Addison disease (or adrenal autoantibodies) plus either autoimmune thyroid disease or type 1 diabetes or both (or their associated autoantibodies).
Diagnostic relationships and common associations in APS I and APS II. The solid lines indicate diagnostic relationships. The dashed lines indicate common associations. The diagnosis of APS I depends on the coexistence of Addison disease (or adrenal autoantibodies) plus either hypoparathyroidism or chronic mucocutaneous candidiasis or both. The diagnosis of APS II depends on the coexistence of Addison disease (or adrenal autoantibodies) plus either autoimmune thyroid disease or type 1 diabetes or both (or their associated autoantibodies).
Clinical aspects
APS I
The major disease components, frequencies, and differences between APS I and II are shown in Table 20-1. Although the disease is not common, the largest cohorts of patients have been reported from Finland, the United States, and among Iranian Jews.51,52,54,60,61 The Finnish cohort of 91 subjects is the largest and most well characterized APS I group of patients in the world.52
Persistent mucocutaneous candidiasis is usually the first sign (60% of all APS I patients) and usually appears during the first year or two of life. In the Finnish cohort, 50% developed candidiasis by age 5 years, 94% by age 20 years, and 100% by 40 years of age.52 All patients with refractory mucocutaneous candidiasis should be thoroughly investigated not only for a T-lymphocyte abnormality (absolute lymphocyte count, enumeration of T-cell subpopulations, assessment of T-cell function) but also for the presence of a polyendocrinopathy. Candidal infections in the diaper area are found early in life, with vulvovaginal candidiasis developing at puberty in females. Colonization of the gut by Candida can lead to intermittent abdominal pain and diarrhea. Infection of the nails with chronic candidiasis may lead to a darkened discoloration, thickening, or erosion. Retrosternal pain in patients with oral candidiasis suggests esophageal candidiasis and can be confirmed by esophagoscopy. Chronic oral mucosal candidiasis must be treated aggressively as candidiasis associated carcinoma of the oral mucosa or esophagus was reported in 7 of the 55 Finnish APS I patients over 25 years of age (note that 5 of the 7 were also smokers).51,62
Oral mucous membranes must therefore be protected from exposures that can increase susceptibility to candidal infections. Specifically, patients should be advised to avoid hard, sharp, or spicy foods, as well as whitening toothpastes or abrasives. Dentures or orthodontics can provide additional surfaces for Candidal growth.51 Data suggest that prolonged therapy with fluconazole, ketoconazole, and miconazole leads to reduced azole susceptibility.63,64 As such, azole therapy should be limited to two to three courses per year utilizing two polyene antifungals. Specifically, a 1- to 2-minute swish with 2 mL of nystatin followed by an amphotericin B lozenge that is allowed to dissolve without chewing should be given four times daily for 4 to 6 weeks.51,64 When candidiasis is recurrent, pulse prophylaxis should follow using a 1-week course of either polyene (as described previously) every 3 weeks. Alternatively, 1 week of twice-daily chlorhexidine mouth rinse has also been used as prophylaxis.51 If topical therapy fails, a 1-week course of high-dose fluconazole (200 to 300 mg in adults) is typically efficacious, though intravenous antifungal therapy may occasionally be required.
Hypoparathyroidism is typically the first endocrinopathy to develop in APS I and eventually occurs in more than 85% of patients. Hypoparathyroidism usually presents after the onset of mucocutaneous candidiasis but before puberty with 33% of APS I patients diagnosed with hypoparathyroidism by 5 years, 66% by 10 years, and nearly 85% by age 30. Severe hypocalcemia as evidenced by seizures, carpopedal spasms, muscle twitching, and laryngospasm may be presenting features of APS I, although these symptoms may be masked in the presence of adrenal insufficiency. Hypocalcemia, hyperphosphatemia and a low intact parathyroid hormone (PTH) level are diagnostic of hypoparathyroidism. Standard therapy consists of calcium salts and activated vitamin D (see Chapter 18 for management). Although data suggest that twice-daily administration of synthetic parathyroid hormone may provide optimal therapy,65 this approach has not yet been approved by the U.S. Food and Drug Administration (FDA).
Autoimmune adrenocortical insufficiency (Addison disease) is the third major component of APS I and typically occurs after mucocutaneous candidiasis and hypoparathyroidism have been diagnosed. Over 85% of APS I patients will eventually develop adrenal insufficiency. Unfortunately, adrenal insufficiency is often initially missed clinically with the diagnosis commonly made late or at the time of a life-threatening adrenal crisis. In the Finnish cohort of APS I patients, 40% had Addison disease by 10 years and nearly 80% by 30 years.52 Deficiencies of cortisol, aldosterone, and adrenal androgens may present simultaneously or may evolve over months to years. The initial symptoms of adrenal insufficiency are often nonspecific, mimicking psychiatric or gastrointestinal disease. These include fatigue, weight loss, myalgias, arthralgias, behavioral changes, nausea and vomiting, abdominal pain, and diarrhea. Over time, hyperpigmentation (due to elevated adrenocorticotropic hormone [ACTH]) in non–sun-exposed areas and postural hypotension can usually be found on careful examination. Unexplained hypotonic dehydration should raise the suspicion of Addison disease. Adrenal crises with hyponatremia, hyperkalemia, acidosis, and hypoglycemia may be fatal unless recognized and treated appropriately. As discussed in detail further, adrenal autoantibodies are used to predict adrenal cortical failure. If present, morning cortisol and renin measurements as well as ACTH stimulation testing may be used diagnostically in asymptomatic patients.
Autoimmune gonadal failure occurs in over 50% of women with APS I by age 20 years; less than 25% of males develop testicular insufficiency.52 Gonadal failure often presents with primary amenorrhea in young women, though menstrual irregularities, polycystic ovaries, or infertility may be presenting features.52,66 As with autoimmune adrenalitis, gonadal failure can be predicted by the presence of steroidal cell autoantibodies.67
Ectodermal dystrophy unrelated to hypoparathyroidism or mucocutaneous candidiasis has been extensively documented in the Finnish cohort. Dental enamel hypoplasia of permanent (but not deciduous) teeth as well as nail dystrophy is commonly found. There may be complete absence of the enamel or transverse hypoplastic bands alternating with zones of well-formed enamel. Dystrophy of nails is manifest by 0.5 to 1 mm pits. Nearly a third of the Finnish patients also had calcification of the tympanic membranes,68 and 20% to 25% develop keratitis.52
As shown in Table 20-1, and in contrast to patients with APS II, type 1 diabetes and thyrogastric autoimmunity (a descriptive term for the combination of autoimmune thyroid disease and atrophic gastritis) are associated with APS I but occur far less frequently than in APS II. When present, thyroiditis is typically atrophic rather than goitrous. Gastric-parietal cell autoimmunity, which leads to atrophic gastritis with resultant achlorhydria and intrinsic factor deficiency, typically presents as iron deficiency anemia or vitamin B12-deficient pernicious anemia. Whereas iron deficiency anemia is microcytic and vitamin B12-deficient anemia is macrocytic, combined iron and vitamin B12 deficiency can be normocytic. It is also important to recognize that the spinal cord consequences of vitamin B12 deficiency can occur in the absence of anemia. Atrophic gastritis occurs in 15% to 30% of APS I cases with a mean age of onset of 16 years.52,68
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree