Thymomas and thymic tumors
Ronan J. Kelly, MD  Alberto M. Marchevsky, MD
Alberto M. Marchevsky, MD
Overview
Thymomas, carcinomas, and other thymic neoplasms are rare and comprise fewer than 1.5% of all solid tumors. They are currently classified according to the recently updated (2015) histologic classification of the World Health Organization (WHO). The histologic features and classification criteria for thymomas and thymic carcinomas are reviewed in this chapter, with an emphasis on various diagnostic problems. Thymomas are staged according to the Masaoka–Koga system, although the American Joint Commission on Cancer (AJCC) is currently developing staging guidelines. There is some current uncertainty regarding how to stage thymic carcinomas. Thymoma patients with stages I and II are treated with radical thymectomy, whereas radiation therapy and chemotherapy are used in patients with more advanced stages. This chapter discusses in detail the current state of the art for the treatment of thymomas and other thymic neoplasms. The results of various therapeutic options are difficult to evaluate with certainty in the absence of a significant number of randomized clinical trials. Indeed, level I and II evidence is difficult to gather for patients with neoplasms that are rare, have a long natural history, and require follow-up for many years. The efforts of the International Thymic Malignancies Interest Group (ITMIG) to develop evidence-based guidelines for the diagnosis, staging, and treatment of thymic neoplasms are described.
Introduction
Thymic tumors are rare and comprise only 0.2–1.5% of all solid tumors or 0.13 per 100,000 person years in the United States.1 As a result, traditional clinical research has been challenging in these tumors. A number of obstacles have also hindered our progress over the past few decades. One of the most compelling has been the lack of an International consensus surrounding appropriate histopathological and staging criteria. At least 15 different stage classifications have been proposed and used.2 This has undoubtedly led to confusion among investigators and made it difficult to compare one clinical trial to another. To date, the most widely used staging classification systems have been the Masaoka and the subsequent Koga modification.3, 4 The vague wording and difficulty of interpretation in using these systems have hampered clinical research and made it difficult to collaborate on an International level. An ongoing effort by the International Thymic Malignancies Interest Group (ITMIG) and the International Association for the Study of Lung Cancer (IASLC) to develop a tumor, node, metastasis (TNM)-based staging system for thymic malignancies should lead to an official, stage classification system as proposed by the American Joint Committee on Cancer (AJCC) and the Union for International Cancer Control (UICC).
The World Health Organization (WHO) histological classification distinguishes thymomas (types A, AB, B1, B2, and B3) from thymic carcinomas (previously designated by WHO as type C) based on the morphology of epithelial tumor cells (with increasing degree of atypia along the spectrum from type A thymomas to thymic carcinomas), the proportion of lymphocytic involvement and resemblance to normal thymic tissue. Incremental improvements in our understanding of the molecular biology of both thymomas and thymic carcinomas have occurred in recent years, thanks to advanced technologies such as comparative genomic hybridization (CGH), expression array analysis, and next generation sequencing. It is becoming more apparent that the subclassifications of types A, AB, B1, B2, B3, and thymic carcinoma have different molecular features that may be clinically relevant. Genomic profiling distinguishes type B3 thymoma and thymic carcinoma as distinct entities from type A and type B2 thymoma. Furthermore, type B2 thymomas can be separated from other subgroups in that it has a more distinctly lymphocytic component than the other groups where epithelial cells predominate.5 Next generation RNA sequencing has recently identified a large microRNA cluster on chromosome 19q13.42 in type A and type AB thymomas, while it is absent in type B thymomas and thymic carcinomas. This cluster has been shown to result in activation of the PI3K/AKT pathway, which suggests a possible role for PI3K inhibitors in these subtypes.6 The presence of KIT mutations in thymic carcinomas is also well described, and although relatively rare, they can be successfully targeted with small molecules.
Surgical resection continues to be the cornerstone of therapy for early-stage disease while a multidisciplinary approach incorporating surgery, radiation, and chemotherapy is recommended in advanced or recurrent disease. In addition, intense interest is now focused on targeting the immune system in these most intriguing of tumors. Thymomas have been known to be associated with a variety of autoimmune disorders such as myasthenia gravis (MG), which have been linked to T-cell-mediated autoimmunity. Processes such as failure of positive and negative selection of T lymphocytes and defects in the autoimmune regulator gene (AIRE) have been proposed as theories underlying autoimmunity but additional research is required. Studies utilizing checkpoint inhibitors, for example, PD-1 inhibitors, are planned in the near future and immunotherapeutic strategies may hold promise for those patients with chemoresistant disease.
This chapter discusses the new histological and staging systems that are being developed and highlights some of the molecular biology breakthroughs that are improving our understanding of these rare tumors. In addition, we discuss the treatment of thymic malignancies focusing on surgery, radiotherapy (RT), and systemic therapy. Recent clinical trials involving chemotherapy and targeted therapeutics are emphasized and future targets are explored.
Incidence and epidemiology
Although thymic malignancies are relatively rare, they are among the most common mediastinal primary tumors with up to 50% of anterior mediastinal masses proving to be of thymic descent.7 Males have a slightly higher risk of developing thymomas than females, and the risk rises with age, reaching a peak in the seventh decade of life, which is in direct contrast to the progressive involution of the thymus with age.8 Data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program collected for Hispanic and Asian/Pacific Islander subgroups has been available only since 1992 and 1998, respectively. Thymoma incidence in the United States is higher in African-Americans and especially Asian/Pacific Islanders than among whites or Hispanics. Furthermore, thymoma arises at a younger age among African-Americans than among whites (median age at diagnosis, 48 years vs 58 years; SEER data). Similarly, MG may be more common in African Americans than in whites.9 Compared with controls, patients with thymoma are more likely to have an autoimmune disease at some point during their lives (32.7% vs 2.4%; P < 0.001), most frequently MG (24.5%), systemic lupus erythematosus (2.4%), or red cell aplasia (1.2%).10 Ethnic variations in terms of higher incidence rates and younger age at diagnosis suggest a role for genetic factors. The distribution of alleles at the HLA locus on chromosome 6 varies markedly across racial groups.8 Both class I and class II HLA proteins are highly expressed on thymic epithelial cells.11 Further research is needed to understand whether particular genetic variants (at HLA or other loci) predispose to thymoma. There are no known etiologic factors for thymic malignancies, although a few cases have developed following radiation therapy to the chest. Epstein–Barr virus (EBV) has been associated with thymic lymphoepithelioma, a rare variant of thymic carcinoma that is usually more frequent in young patients.12 A few patients with the multiple endocrine neoplasm type I syndrome (MEN I) have had associated thymomas or neuroendocrine carcinomas of thymic origin.
Anatomic pathogenesis
Embryology and anatomy
The thymus is embryologically derived from the endodermal epithelium of the third pharyngeal pouches (which also give rise to the lower pair of parathyroid glands) and, less constantly, the fourth ones as well.13, 14 The right and left thymic anlagen migrate downward into the anterosuperior mediastinum, joining together without complete fusion to form a bilobate organ. Although most thymic tumors are located in the anterosuperior mediastinum, variations in migration account for the findings of gross or microscopic thymic tissue anywhere between the hyoid bone superiorly and the diaphragm inferiorly. Wide exposure of the mediastinum and even the neck is therefore necessary if surgical removal of the entire thymus is indicated, as in patients with thymoma or those with MG (with or without associated thymoma). The absolute weight of the thymus reaches its peak in the pubertal years (mean 34 ± 15 g between age 10 and 15 years) and then gradually decreases, although this age-related involution normally is never complete. Histologically, the normal thymus shows distinctive lobules with a sharp demarcation between the cortex, rich in lymphocytes, and the medulla, rich in epithelial cells and characteristic Hassall’s corpuscles, formed by concentric layers of mature epithelial cells. The thymus plays a critical role in the maturation of bone marrow-derived lymphocytes into T cells and, as such, in cell-mediated immunity. It has a rich blood supply but no afferent lymphatics. Efferent lymphatics apparently originate from perivascular spaces and drain into the mediastinal and lower cervical nodes.
Thymic neoplasms
The thymus can develop a variety of epithelial, germ cell, lymphoid, mesenchymal, and other neoplasms listed in Table 1.13
Table 1 Primary thymic neoplasms
Thymic epithelial tumors
|
Germ cell tumors
|
Mediastinal lymphomas
|
| Histiocytic and dendritic cell tumors |
| Myeloid sarcoma |
Mesenchymal tumors
|
Pathology of thymic epithelial neoplasms
Thymic epithelial neoplasms include low-grade malignant lesions, designated as thymomas and malignant lesions of moderate to high-grade malignant potential classified as thymic carcinomas.13, 15, 16
Pathology of thymomas
Thymomas appear grossly as single or multiple, usually encapsulated neoplasms that range in size from microscopic lesions to large tumors replacing the entire anterior mediastinum and compressing the adjacent intrathoracic structures.13, 17–21 Grossly, most thymomas are well encapsulated and limited to the thymic gland (Figure 1) but some lesions are locally invasive extending into mediastinal soft tissues, superior vena cava and other vascular structures, lymph nodes, pleura, pericardium, trachea, and/or other intrathoracic structures adjacent to the thymus (Figure 2). On section, thymomas exhibit a distinctive fibrous capsule and multiple fibrous septa that divide the lesion into a characteristic lobulated appearance (Figure 3). They are usually solid tumors but can undergo cystic degeneration. Predominantly, cystic thymomas are distinguished from benign thymic cysts by the presence of focal solid areas.
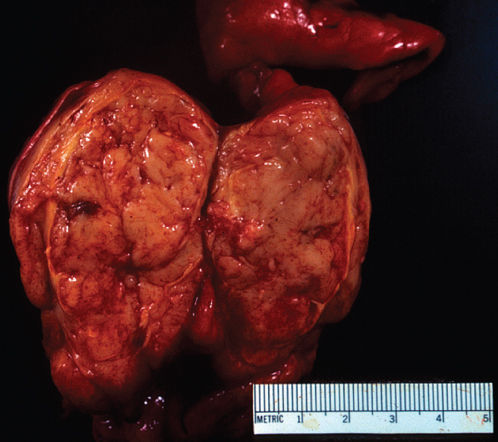
Figure 1 Gross photograph of thymoma showing an encapsulated tumor with a gray, soft surface exhibiting characteristic fibrous septa.
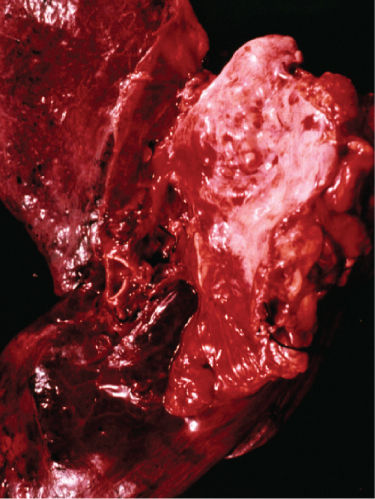
Figure 2 Gross photograph of invasive thymoma showing invasion of mediastinal soft tissues and bronchial wall.
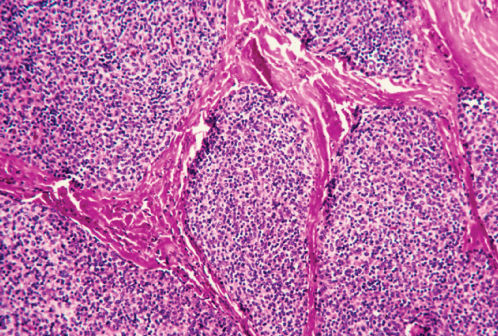
Figure 3 Photomicrograph of thymoma showing the characteristic lobulations of the lesion. The epithelial and lymphoid cells are separated by thin fibrous septa (H&E; ×40).
Microscopically, thymomas are composed of epithelial cells with spindle-, oval-, or polygonal-shaped nuclei admixed with a variable number of mature lymphocytes. The epithelial and lymphoid cells of thymomas are arranged in solid sheets usually divided by fibrous septa in a somewhat lobulated appearance that can be observed at low-power microscopy in most thymomas (Figure 3). Other histologic features of thymomas include perivascular spaces (Figure 4), medullary areas, pseudorosettes, gland-like structures, Hassall’s corpuscles, areas of cystic degeneration, and less frequently hemorrhagic and/or calcified areas. Thymomas are often encapsulated, but can invade the capsule into adjacent tissues (Figure 5). Mitoses, cellular atypia, and necrosis are unusual in thymomas and should raise the suspicion of a thymic carcinoma.
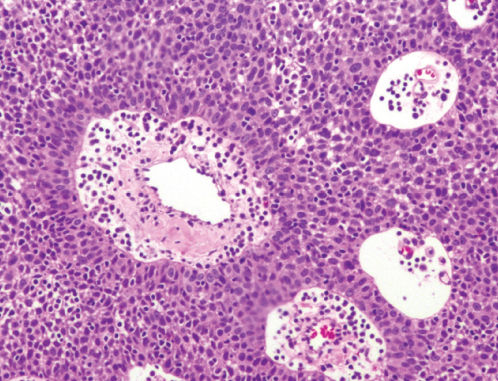
Figure 4 Photomicrograph of thymoma at higher power showing the tumor cells arranged around blood vessels, in a characteristic perivascular growth pattern. The tumor cells are polygonal, with minimal cytologic atypia. They are admixed with small mature lymphocytes (H&E; ×100).
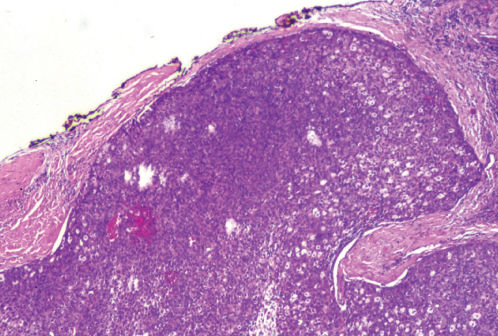
Figure 5 Photomicrograph of a thymoma showing invasion of the capsule (H&E; ×40).
World Health Organization classification of thymomas
Different classification schema have been proposed for the categorization of thymic epithelial neoplasms, but the most widely used classification scheme of these tumors has been proposed by the WHO in 1999 and has been modified in 2004 and 2015 ( Table 2).
Table 2 World Health Organization classification of thymomas and selected morphological features of the neoplasms
| Type A (spindle cell) Atypical type A (spindle cells with focal atypia) |
| Type AB (mixed spindle and polygonal cells) |
| Type B1 (lymphocyte-rich, scattered polygonal cells) |
| Type B2 (lymphocyte-rich with higher density of polygonal epithelial cells and focal, minimal atypia) |
| Type B3 (lymphocyte poor, polygonal cells with mild atypia) Rare thymomas
|
Thymomas A are composed predominantly of spindle epithelial cells with minimal numbers of lymphocytes (Figures 6 and 7).19, 20, 22, 23 Patients with thymoma A have a lower incidence of MG than those with other thymoma histologic types and a greater incidence of aplastic anemia. Thymomas B1–B3 are composed of polygonal epithelial cells that are designated as thymoma B and further subclassified into B1, B2, and B3 lesions. B1 thymomas are composed of inconspicuous polygonal epithelial cells admixed with a large number of mature lymphocytes. These lesions characteristically have scattered round, hypochromatic areas designated as medullary areas and have been described in the past as lymphocyte-predominant thymomas because of the sparsity of visible epithelial cells in histologic sections stained with hematoxylin and eosin (H&E). B2 thymomas are composed of polygonal cells that are more conspicuous than those seen in B1 lesions and frequently exhibit slight nuclear variability and focally prominent nucleoli (Figure 8). B3 thymomas, classified in other schema as “atypical thymoma,” are composed of polygonal or spindled epithelial cells that exhibit moderate variability in cell size and shape (anisocytosis), focal nuclear hyperchromasia, and cytologic atypia. B3 thymomas characteristically have fewer lymphocytes than seen in B1 and B2 lesions and can be difficult to distinguish from low-grade thymic carcinomas. Thymomas AB have >5% lymphocytes and are composed of both spindle epithelial cells and polygonal cells. Thymomas are frequently heterogeneous neoplasms and can exhibit different WHO “types” on different sections taken from the same lesion.24
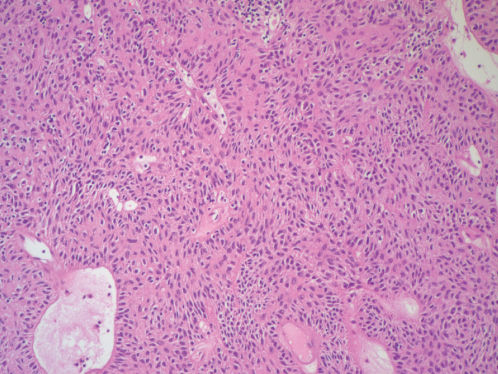
Figure 6 Photomicrograph of spindle cell thymoma (thymoma A). The tumor cells have elongated nuclei. Note the paucity of mature lymphocytes (H&E; ×100).
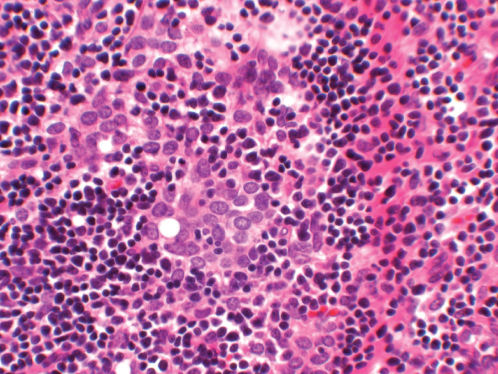
Figure 7 Photomicrograph of thymoma A showing small polygonal epithelial cells with round nuclei, lack of visible nucleoli and scanty cytoplasm. They are admixed with numerous lymphocytes (H&E; ×200).
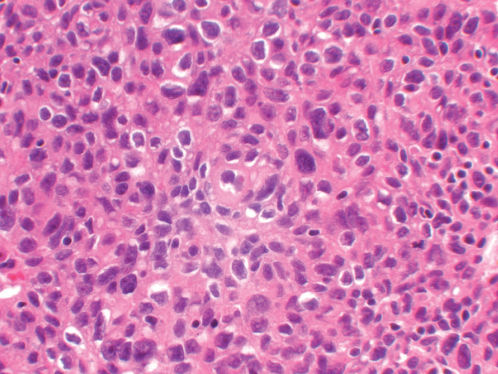
Figure 8 Photomicrograph of thymoma B3 (the so-called atypical thymoma). The tumor cells exhibit considerable variation in size and shape, hyperchromasia and somewhat irregular nuclear membranes. There are relatively few lymphocytes in the background (H&E; ×200).
Immunohistochemistry and other ancillary techniques
The epithelial cells of thymomas exhibit cytoplasmic immunoreactivity to keratin AE1/AE3, a feature that is generally very helpful to confirm the diagnosis of thymoma on needle biopsies and other pathologic materials ( Figure 9). A variety of other epitopes have been shown in the different WHO histologic types of thymomas, such as CD5, laminin, collagen IV, metallothionein, PE-53, cytokeratins such as CAM 5.2, CK7, CK14 and CK18, CD57, and others. However, they have limited diagnostic or prognostic value. CD5 is usually negative in thymomas other than thymoma C. Foxnl, CD205, and desmoglein-3 are novel markers of thymic epithelial cells that may be useful to distinguish different thymomas and thymic carcinoma.
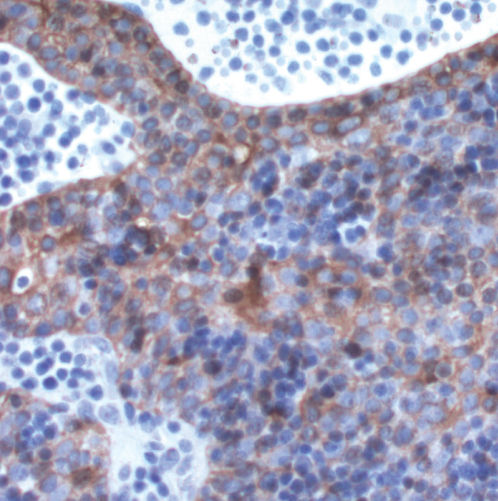
Figure 9 The epithelial cells of thymomas exhibit cytoplasmic immunoreactivity when stained for keratin AE1/AE2 (Immunostain; ×200).
Electron microscopy is seldom used for the routine diagnosis of thymomas.13 The tumor cells reveal ultrastructural features that are characteristic for epithelial differentiation such as desmosomes, tonofilaments, elongated cell processes, and a lack of dense core granules. The malignant epithelial cells form an irregular mesh with multiple communicating spaces and channels containing mature lymphomas and resembling the normal ultrastructural features and microenvironment of the thymus gland.
Histologic prognostic features in thymomas
All thymomas are low-grade malignant neoplasms.24–27 Thymomas A (spindle cell thymomas) have been considered as benign lesions in the past, but these lesions can recur or metastasize in a small number of patients. Patients with thymomas A, AB, and B1 appear to have the best prognosis, while those with thymomas B3 generally have more frequent recurrences and metastases and shorter survival.
Staging systems for thymomas
As it has been controversial whether thymomas are benign or malignant neoplasms, there is no current American Joint Commission on Cancer TNM system for thymomas. The most widely used system for staging these tumors is the Masaoka–Koga staging system given in Table 3.28–32
Table 3 Masaoka–Koga staging of thymomas
| Stage | Extent of disease |
| I | Totally encapsulated |
| II | Microscopic (IIa) or macroscopic (IIb) transcapsular invasion into surrounding fat or mediastinal pleura |
| III | Invasion of surrounding organs (pericardium, lung, and great vessels) |
| IV | (A) Pleural or pericardial implants (B) Embolic metastasis |
Thymic carcinomas
Thymic carcinomas are unusual epithelial tumors comprising approximately 0.06% of thymic neoplasms and composed of epithelial cells that exhibit cytological features characteristic of malignancy, such as considerable pleomorphism, hyperchromasia, prominent nucleoli, increased mitotic activity, and/or necrosis.22, 28, 31, 33–38 A variety of histologic variants, described in Table 4, have been described. Squamous cell carcinomas are probably the most frequent variant of these neoplasms. Lymphoepithelioma of the thymus is a particularly interesting variant of thymic carcinoma frequently associated with the EBV expression and exhibits similar histopathologic features to those seen in the head and neck area.16 Carcinoma with NUT, t(15;19) translocation is a recently described aggressive variant of thymic carcinomas that affects children and young adults.34
Table 4 World Health Organization classification of thymic carcinomas
| Squamous cell carcinoma |
| Basaloid carcinoma |
| Mucoepidermoid carcinoma |
| Lymphoepithelioma-like carcinoma |
| Clear cell carcinoma |
| Sarcomatoid carcinoma |
| Adenocarcinoma |
| NUT carcinoma |
| Undifferentiated carcinoma |
| Other rare thymic carcinomas |
Thymic carcinoid and neuroendocrine tumors
Carcinoid tumors and other neuroendocrine neoplasms given in Table 5 can arise in the thymus.23, 39–43 They are more aggressive than their counterparts in the lung and other organs and with metastases at diagnosis in 30–40% of cases.15 The association with Cushing syndrome with ectopic ACTH production in a patient with a thymic tumor should raise this possibility because it occurs in 30% of patients with thymic carcinoid tumor but not in patients with thymoma. Other paraneoplastic syndromes, such as osteoarthropathy and Eaton–Lambert syndrome, and an association with multiple endocrine neoplasia (type I or II) have been described.39, 43 Carcinoid syndrome has not been reported. Neuroendocrine tumors exhibit histopathologic features at low-power microscopy characteristic of neuroendocrine differentiation, including the formation of cellular nests, trabeculae, and pseudorosettes.13 The tumor cells vary according to the cell type. Carcinoid tumors are composed of round to elongated nuclei with “salt-and-pepper” nuclear chromatin, variable hyperchromasia, and minimal pleomorphism. Typical carcinoid tumors usually lack mitosis and necrosis, while atypical carcinoid tumors exhibit low mitotic activity and focal areas of necrosis. Large cell neuroendocrine carcinoma of the thymus are composed of large, pleomorphic, hyperchromatic cells that can exhibit focally prominent nucleoli. Small cell carcinomas of the thymus are composed of small hyperchromatic cells with nuclear molding and inconspicuous nucleoli. Both variants of high-grade thymic neuroendocrine carcinomas exhibit frequent mitosis, usually higher than 10 mitosis in 10 high-power fields and extensive areas of necrosis. The tumor cells of all variants of thymic neuroendocrine carcinoma exhibit cytoplasmic immunoreactivity to chromogranin and synaptophysin and dense core neuroendocrine granules under electron microscopy. Immunostains for Ki-67 can be used to estimate the proliferative fraction of these lesions.
Table 5 Thymic neuroendocrine tumors
| Typical carcinoid |
| Atypical carcinoid |
| Large cell neuroendocrine carcinoma |
| Small cell carcinoma |
Thymic lymphomas
Lymphomas are, with thymomas, the most common tumors of the thymus.13, 33, 44–46 Although the thymus is a T-cell organ, the most frequent lymphomas of the thymus in adult patients are Hodgkin lymphoma and B-cell lymphomas (Table 3). Primary Hodgkin lymphoma of the thymus tends to be limited to the gland and is, as a rule, of the nodular sclerosis type. It is frequently associated with cystic change in the adjacent thymic tissue. B-cell lymphomas of the mediastinum frequently involve the thymus and lymph nodes and frequently exhibit histologic features that are unusual for B-cell lesions in other locations, such as sclerosis with compartmentalization of the tumor cells, clear cell features, and focal formation of cellular nests simulating an epithelial malignancy. They tend to follow an aggressive clinical course. Lymphomas involving the thymus and mediastinal lymph nodes in children include Burkitt’s lymphoma, lymphoblastic lymphoma, and a lesion of T-cell origin. It is of prime importance to differentiate a lymphoma from a thymoma in view of the different therapeutic approaches. Special stains and electron microscopy may be necessary in difficult cases.
Germ cell tumors of the thymus
The thymus is a classic site of extragonadal primary germ cell tumors (Table 1). The most common ones are seminomas (sometimes difficult to differentiate from thymoma) and teratomas (mature or immature). Embryonal carcinomas, yolk sac tumors, teratocarcinomas, and choriocarcinomas have also been described.
Other thymic tumors
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








