Author
Follow-up (years)
Approach
Patients
Tumor size (cm)
Primary effectiveness (%)
Secondary effectiveness (%)
DFS (5-year %)
OS (5-year %)
CSS (5-year %)
Ma et al. [86]
5 (median)
Mix
52
2.2 (mean)
100
N/A
94.2
95.7
100
Psutka et al. [87]
6.43 (median)
Perc
185
3 (median)
87
100
87.6
73.3
99.4
Ramirez et al. [88]
4.9 (median)
Lap
79
2.2 (median)
97.5
100
93.3
72
100
Best et al. [89]
4.5 (median)
Mix
142
2.4 (median)
95
98
91
nc
nc
Olweny et al. [90]
6.5 (median)
Mix
37
2.1 (median)
94.6
97.3
89.2
97.2
97.2
6.1 (median)
PN
37
2.5 (median)
100
N/A
89.2
100
100
Zagoria et al. [91]
4.7 (median)
Perc
41
2.6 (median)
89.5
100
83
66
97.5
Levinson et al. [92]
5.1 (mean)
Perc
31
2 (median)
87
100
89.2 (6.7 year %)
62.7 (6.7 year %)
100 (6.7 year %)
Table 12.2
Long-term (≥4 year follow-up) cryoablation results
Author | Follow-up (years) | Approach | Patients | Tumor size (cm) | Primary effectiveness (%) | Secondary effectiveness (%) | DFS (5-year %) | OS (5-year %) | CSS (5-year %) |
|---|---|---|---|---|---|---|---|---|---|
Johnson et al. [93] | 8.2 (mean) | Lap | 144 | 2.3 (mean) | 98.6 | nc | 95.4 | 90.5 | 100 |
Georgiades et al. [94] | 5 (mean) | Perc | 134 | 2.8 (median) | 98.5 | 99.3 | 97 | 97.8 | 100 |
Tanagho et al. [95] | 6.3 (mean) | Lap | 62 | 2.5 (mean) | 100 | N/A | 80 | 76.2 | 100 |
Aron et al. [96] | 7.8 (median) | Lap | 55 | 2.3 (mean) | 100 | N/A | 86 | 84 | 92.5 |
CA experience has been greatest using the laparoscopic approach, although long-term percutaneous CA series are also available. Johnson et al. reported on their experience with laparoscopic CA in 144 patients followed for an average of 8.2 years. They reported 5-year DFS of 95.4 %, OS 90.5 %, and CSS 100 % [93]. Georgiades and colleagues reported on their cohort of 134 patients treated with percutaneous CA followed for 5 years. The 5-year DFS was 97 %, OS was 97.8 %, and CSS was 100 % [94]. Tanagho et al. followed 62 patients for a mean of 76 months after laparoscopic CA and found a 6-year DFS of 80 %, OS 76.2 %, and CSS of 100 % [95]. Aron et al. reported 5-year disease-free survival of 81 % in 55 patients with biopsy-proven RCC at a median follow-up of 93 months [96].
Though these long-term data give one greater confidence in the efficacy of thermal ablation for RCC, continued follow-up of these cohorts is necessary because of the known indolent growth rates of small RCCs.
12.7 Post-procedure Follow-Up
Follow-up should encompass an assessment of the patient’s clinical status including renal function as well as a review of imaging looking for delayed complications and residual, recurrent, or metastatic disease. A clinic visit should be arranged in the weeks after the procedure to assess for pain, urinary symptoms, fever, or chills. The skin entry sites should be examined.
Given that ablative therapy is advocated in those with limited renal reserve, it is important that the impact of ablation on renal function if any be recorded. Lucas et al. examined the impact of RFA, partial nephrectomy, and radical nephrectomy on renal function in patients with small renal masses (<4 cm). The mean pretreatment GFR was 73.4, 70.9, and 74.8 mL/min/1.73 m2 in the RFA, partial nephrectomy, and radical nephrectomy groups. Following intervention, the 3-year freedom from stage 3 CKD was 95.2 % for RFA, 70.7 % for partial nephrectomy, and 39.9 % for radical nephrectomy (p < 0.001). Patients undergoing radical and partial nephrectomy were 34.3 (p = 0.001) and 10.9 (p = 0.024) times more likely, respectively, to develop stage 3 CKD compared to RFA counterparts [97]. In patients with a solitary kidney, Raman et al. examined the impact of RFA on renal function in 16 patients with 21 small renal masses (<=4 cm). In this series, the mean preoperative GFR of 54.2 mL/min/1.73 m2 declined only to 47.5 mL/min/1.73 m2 at the last follow-up (mean follow-up of 30.7 months). Patients treated with open partial nephrectomy had a greater decline in GFR compared with those who underwent RFA, at all post-procedure times evaluated: 15.8 % versus 7.1 % at 0–3 months, 24.5 % versus 10.4 % at 12 months, and 28.6 % versus 11.4 % at the last follow-up (p < 0.001 for all time periods) [34].
There is no standardized follow-up algorithm for ablated renal tumors. The follow-up imaging interval varies among institutions. Matin et al. detected 70 % of incomplete treatments within the first 3 months of treatment. They recommended at least three to four imaging studies in the first year after ablative therapy: months 1, 3, 6 (optional), and 12 [98]. Ideally, follow-up should be performed using the cross-sectional imaging modality used to perform the ablation. Persistent nodular enhancement in the ablation zone up to 3 months post treatment is worrisome for residual disease [99]. Differential diagnosis includes inflammation or volume averaging. Recurrent disease is suspected if the ablation zone is enlarging on serial scans and/or nodular contrast enhancement that was not present on the initial post-ablation study is identified [99]. The renal vein and IVC should be assessed for evidence of enlargement or abnormal enhancement. A search for a new primary tumor and metastatic disease should be performed. Classically, the RFA zone has a “bull’s-eye” appearance on surveillance imaging – non-enhancing soft tissue surrounded by enhancing normal renal parenchyma [99]. The ablation zone is usually T2 hypointense compared with the normal renal parenchyma and can have variable intensity on T1-weighted sequences [100, 101]. Subtraction of post-gadolinium and non-contrast T1-weighted data may enhance detection of subtle foci of residual or recurrent disease [102]. While hemorrhage can artificially increase the size of the ablation zone on the immediate post-procedure scan, the lesion should slowly involute to pre-RFA size on serial scans [103] (Figs. 12.1 and 12.2).
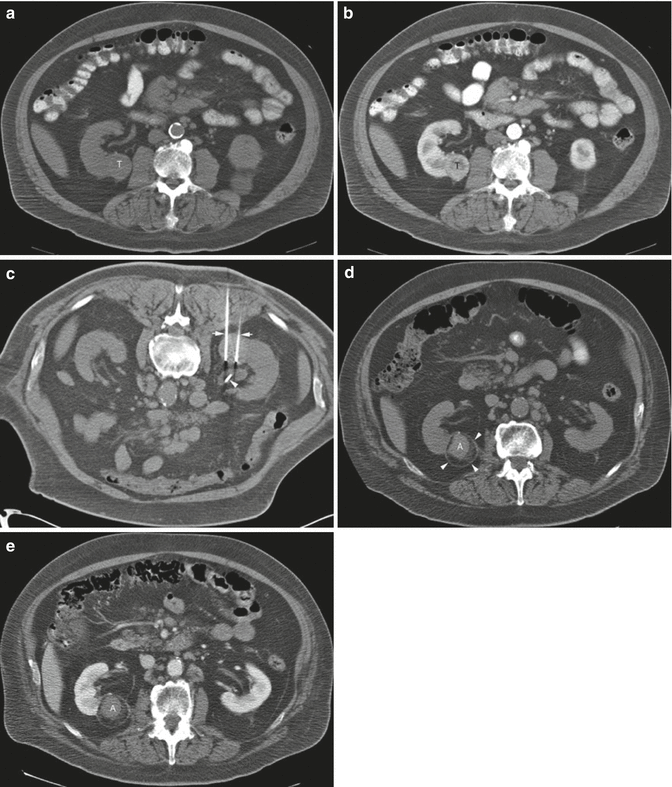
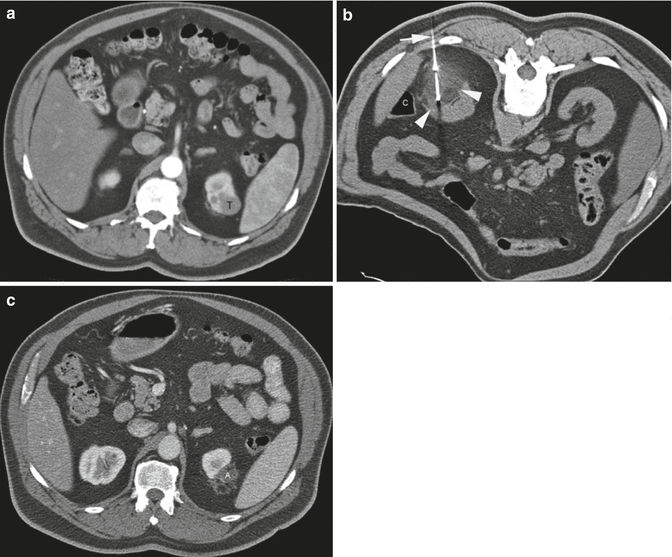

Fig.12.1
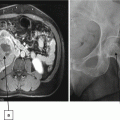
A 68-year-old man was found to have a 3.2-cm solid enhancing mass in the right kidney. Biopsy showed renal cell carcinoma, clear cell type. (a) Axial CT image of the abdomen without contrast medium shows a tumor (T) along the medial border of the right kidney. (b) After administration of iodinated contrast medium, the tumor (T) shows marked enhancement. (c) Axial CT image of the patient in prone position shows two radiofrequency electrodes (arrows) entering the tumor from a posterior approach. The tip of each electrode is carefully positioned at the anterior margin of the tumor. A retrograde ureteral catheter (arrowhead) was placed for continuous infusion of cold fluid to prevent heating injury to the ureteropelvic junction. Four overlapping ablations were performed to completely ablate the tumor. (d) Axial CT image of the abdomen without contrast medium 30 months after ablation shows a soft tissue density at the center of the ablation zone (A) surrounded by a fibrous capsule (arrowheads). The capsule has engulfed retroperitoneal fat into the ablation zone. (e) After administration of the contrast, there is no enhancement of the ablation zone (A). A biopsy of the ablation zone (not shown here) demonstrated necrotic tissue and no viable tumor

Fig. 12.2
A 62-year-old man underwent CT examination for staging of prostate cancer. He was found to have a 2.7-cm enhancing mass at the upper pole of his left kidney. Biopsy showed renal cell carcinoma, papillary type 1 and Fuhrman nuclear grade 2. (a) Axial CT image of the abdomen after administration of contrast shows the tumor (T) involving the upper pole of the left kidney. (b) Axial CT image of the abdomen in prone position shows one of the three cryoprobes (arrow) placed into the tumor from a posterior approach under CT guidance. The ice ball has a lower density compared to the normal kidney. The edge of the ice ball is sharply demarcated at its boundary with normal renal parenchyma. Monitoring the size and extent of the ice ball with CT intermittent CT imaging helps avoid thermal injury to the adjacent structures such as the colon (C). (c) Axial CT image of the abdomen with iodinated contrast 17 months after ablation shows involution of the ablation zone (A) with minimal residual non-enhancing necrotic tissue
During CA, the tumor is frozen and is identified by a well-defined area of low attenuation on CT and is both T1 and T2 hypointense on MRI. While the cryoablated zone is typically non-enhancing on CT and MRI surveillance studies, residual contrast enhancement has been reported [104–106]. In a review of 32 lesions treated with laparoscopic CA, Stein et al. identified persistent ablation site enhancement in 15.6 % (5/32) at 3 months, three of which persisted at 6 months and one displayed enhancement at 9 months. The latter underwent partial nephrectomy that demonstrated no recurrent cancer [104]. The ablation zone is frequently isointense on T1-weighted sequences and hypointense on T2-weighted sequences relative to the renal parenchyma. Involution of the tumor mass on surveillance studies is more prominent following CA due to tissue resorption, than with RFA where the lesion is replaced by scar tissue [99]. Gill et al. reported that tumor size decreased an average of 75 % 3 years post ablation. A further 38 % of cryoablated tumors were not detectable by MR imaging at 3 years (Fig. 12.3) [107].
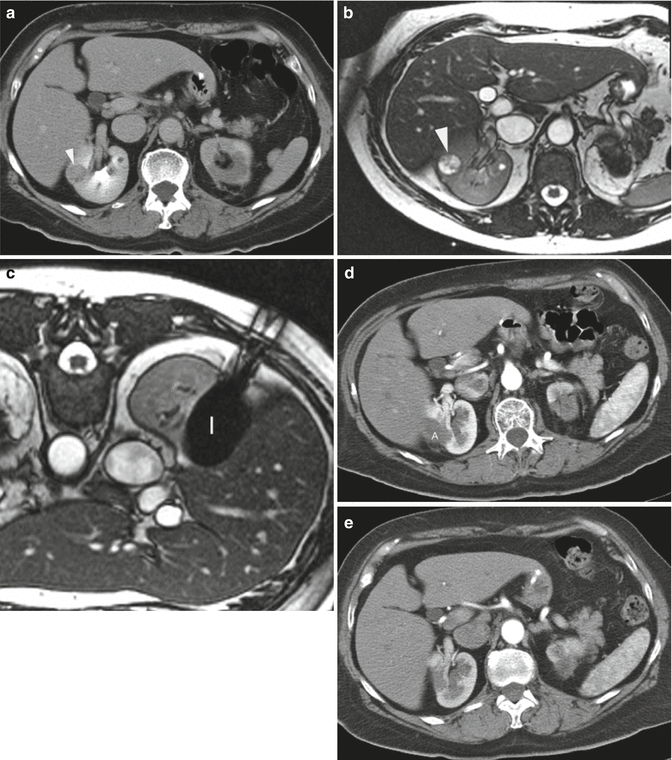

Fig. 12.3
A 65-year-old woman underwent CT imaging for the workup of pancreatic cysts. She was found to have bilateral renal tumors. Biopsy showed renal cell carcinoma, clear cell type and Fuhrman nuclear grade 2 on the right and 1 on the left. Genetic analysis was negative for VHL. The left upper pole renal tumor (not shown here) was treated with percutaneous ablation. (a) Axial CT image of the abdomen after administration of IV contrast shows a solid mass (arrowhead) in the lateral mid-pole of the right kidney. The tumor was not easily seen on CT images without contrast. (b) Axial T2-weighted MRI shows the tumor as a bright, hyperintense lesion (arrowhead). She underwent MRI-guided cryoablation of her right renal tumor. (c) Axial T2-weighted MR image of the patient in prone position shows the ice ball (I) covering the entire tumor. (d) Axial contrast-enhanced CT of the abdomen 3 months after ablation shows the ablation zone (A) as non-enhancing soft tissue. (e) Follow-up CT study at 22 months shows complete resorption of the ablated tumor
When recurrence is suspected on follow-up imaging, further management options include active surveillance, repeated ablations, and surgical extirpation. Given that the mean growth rate of small renal masses is 0.13 cm per year, surveillance is reasonable [108]. The majority of recurrences are managed with repeat ablation. Between 7.4 % and 8.5 % of all RF lesions and 0.9 % and 1.3 % of all CA lesions are reablated [61, 109]. In a review of 337 CA patients and 283 RFA patients, Long et al. reported reablation rates of 2.5 % for those who underwent percutaneous CA, 8.8 % for those who underwent percutaneous RFA, and 0 % for those treated with laparoscopic RFA or CA [109]. The inferior results observed with RFA may relate to the inability to precisely monitor treatment efficacy during the procedure compared with CA and perhaps a lower threshold to repeat the percutaneous ablation in the presence of suspicious imaging results. In addition, larger applicators and their placement under direct vision are possible with a laparoscopic approach. Repeat ablations may be performed laparoscopically or percutaneously, although repeat laparoscopic intervention is more challenging. Matin et al. reported 4.2 % incidence of local disease progression after repeat ablations at 2-year follow-up [98]. Salvage nephrectomy is reserved for those in whom reablations have failed or the tumor is too large for reablation. While a surgical resection may be technically feasible, intraoperative and postoperative complications are greater [110].
12.8 Complications
Complications following energy ablation of a renal mass are infrequent and have an incidence of 3–12 % [52, 111–114]. Johnson et al. reviewed complications following 271 RF and CA procedures, both percutaneous and laparoscopic, performed at four institutions. A total of 30 complications (11.1 %) occurred including 5 major (1.8 %), 25 minor (9.2 %), and 1 death (0.4 %). Major and minor complication rates were 1.4 % and 12.2 % for CA and 2.2 % and 6 % for RFA [112]. Atwell and colleagues reported their single institution with 573 percutaneous RFA and CA procedures. They reported 63 overall complications (11 %) including 38 (6.6 %) major complications and no deaths. Major complication rates were 8.4 % for CA and 4.7 % for RFA, while minor complication rates were 4.8 % for CA and 5.1 % for RFA [114].
Ablation-related injuries are either mechanical or thermal. Structures that are at greatest risk of injury are nerves, vessels, the renal collecting system, and adjacent bowel. Hemorrhage is the most common major complication and is more commonly associated with CA [112, 114]. It usually arises from direct mechanical injury to a vessel by the applicator. The risk is greater with centrally located tumors in which the applicator may traverse numerous segmental vessels en route to the lesion. Bleeding requiring transfusion has been reported in <1 % of RFA and 4.9 % of CA cases. In a review from Lehman et al., major hemorrhage accounted for over 60 % of complications in lesions over 3 cm in size treated via laparoscopic CA [64]. In a retrospective review of 108 percutaneous CAs of lesions over 3 cm, Schmit et al. reported an 8 % major complication rate. Significant hemorrhage following removal of the cryoprobes from the ablated tumor occurred in four of the six patients who sustained a major complication [115]. Cracking of the ice ball with associated parenchymal injury is a recognized, albeit uncommon complication of CA that can result in significant hemorrhage [116, 117]. Potential risk factors include the use of larger-diameter and/or multiple CA probes, initiating a second adjacent ice ball after the primary ice ball had already been formed, and removal of the CA probes before the ice ball has completely thawed [116, 117]. If hemodynamic stability cannot be restored with conservative measures, trans-arterial embolization may be required. Massive hemorrhage due to an arteriovenous fistula is rare but has been described [118]. Bleeding may be avoided by ensuring that coagulopathies and thrombocytopenia are corrected in advance, antiplatelet and anticoagulant agents are held for an appropriate period prior to the procedure, and patient movement is minimized with adequate sedation. Continuous monitoring of the applicator during placement using ultrasound or CT fluoroscopy, ensuring the applicator position is stable before ablation is commenced, can help to minimize hemorrhage. In addition, pre-procedure arterial embolization might also help reduce hemorrhage after CA [73]. Ultrasound or CT imaging of the kidney should be performed at the end of the procedure to rule out bleeding. If ureteral or urethral obstruction with clots occurs, ureteric stenting and/or urinary catheter placement with bladder irrigation may be required.
The incidence of direct thermal injury to the ureter, usually with RFA, has been reported at 1–2 % [52, 111, 114]. Tumors located in the medial aspect of the lower pole are at greatest risk of injury due to their close proximity to the ureter. The risk of ureteral stricture is increased when the distance between the tumor and ureter is less than 2 cm [119]. Retrograde pyeloperfusion using a chilled dextrose solution can help avoid injury during ablation [44, 75, 76]. The trade-off may be suboptimal ablation due to heat sink from the adjacent fluid. CT urography should be performed following ablation if an injury is suspected. The injury can manifest radiologically as ureteral wall thickening, periureteral fat stranding, hydronephrosis, or urinoma. If not promptly identified, acute renal failure can ensue.
Perinephric fat thickness less than 5 mm between the tumor and the bowel is associated with increased risk of thermal injury to the bowel. The risk is greatest with lower pole anterior lesions. Bowel wall thickening is the most likely finding on immediate post-procedure CT. In the weeks after the procedure, the bowel may become adherent to the kidney. Long-term serious sequelae include stricture, obstruction, and perforation. Adjuvant techniques to avoid bowel injury are described in Sect. 12.5.
Pneumothorax has an incidence of 2 % [111]. The risk is greatest with upper pole RCC in which the lung base overlies the proposed electrode trajectory. The majority of cases can be managed conservatively. Moderate to severe pneumothoraces or those associated with new respiratory symptoms may require aspiration and possible chest tube placement. Seeding of the needle track is extremely rare, and enhancing nodules along the needle track often represent inflammatory nodules [120–122].
12.9 Conclusion
Partial nephrectomy remains the gold standard for the treatment of RCC. However, RFA and CA have been shown to be safe and effective treatment options in a select patient population. While the future of these minimally invasive therapies appears promising, the interpretation and validation of the data that exists are fraught with difficulty. Standardization of reporting criteria including clearly defined treatment outcomes and pretreatment histological proof of disease are required to better define the long-term oncologic efficacy of thermal ablation therapies.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree