Symptoms or signs
Skinner n = 309 (%)
Gibbons n = 110 (%)
Jayson N = 131 (%)
Gupta N = 811 group 1 group 4
Hematuria
59
37
24
29
39
Abdominal or flank mass
45
21
8
49
22
Pain
41
21
10
28
13
Weight loss
28
30
25
16
Symptoms from metastasis
10
–
–
–
–
Classic triad
9
–
–
–
–
Acute varicocele
2
–
–
–
–
Incidental finding
7
40
61
–
–
The classic triad described in RCC is comprised of hematuria, flank pain, and fever but is seen in only 9 % of patients. Clinical presentation is actually extremely variable and is highly dependent on stage of presentation. The sequestered location of the kidney, in the retroperitoneum, results in asymptomatic and non-palpable masses that present only at an advanced or metastatic stage. Incidental detection has increased over time. Between 1935 and 1965, 7 % of tumors were discovered incidentally. In a National Cancer Institute (NCI) study conducted in metropolitan Detroit and Chicago from 2002 to 2007, the proportion of asymptomatic cases increased from 35 % in 2002 to 50 % in 2007. Cases before 1973 were found without the benefit of computed tomography (CT) or ultrasound scanning, whereas those after 1980 were discovered largely because of the widespread use of these technologies. What was once an internist’s tumor has transformed into a radiologist’s tumor. Incidental tumors diagnosed at an earlier stage obviously have a better prognosis. In a recent single-institution study, patients who underwent surgical resection from January 1, 1988, and December 31, 2007, were reviewed. Data were divided into four periods, with each time period encompassing 5 years. Over time the rate of incidental detection increased, from 10.6 to 27.6 % [1] largely because of imaging for evaluation of vague abdominal symptoms (see Fig. 7.1). The incidental tumors are more likely to be smaller (<4 cm) and have a lower grade (Fuhrman grade 1–2), which contributes to better cancer-related prognosis. The incidental finding of renal mass can also induce morbidity and the risks vs. benefits of therapy should be carefully considered, especially in cases with significant comorbidities (Table 7.2).
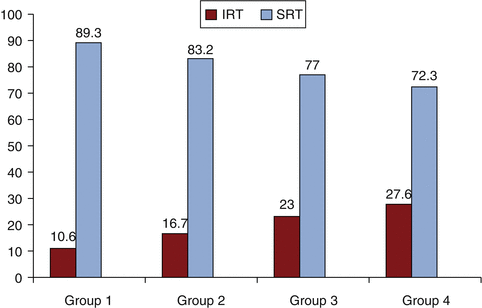
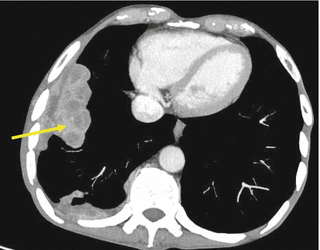

Fig. 7.1
Comparison of incidental and symptomatic presentation of RCC from different time periods. Group 1:1988–1992; Group 2:1993–1997; Group 3:1998–2002; Group 4: 2003–2007 (Reprinted with permission. Medknow Publications & Media Pvt. Ltd. All rights reserved. Gupta et al. [1])

Fig. 7.2
Pulmonary metastasis in a patient with clear cell RCC. Yellow arrow shows sites of lung metastases from renal cancer
Table 7.2
Paraneoplastic manifestations of RCC: incidence and prognostic significance
Type | Incidence (%) | Prognostic significance |
|---|---|---|
Endocrine | ||
Hypercalcemia | 13–20 | Unfavorable |
Hypertension | 40 | – |
Polycythemia | 1–8 | – |
Stauffer’s syndrome | 3–20 | Unfavorable |
Elevated alkaline phosphatase | 10 | Unfavorable |
Cushing syndrome | 2 | – |
Thrombocytosis | – | Unfavorable |
Cachexia | 30 | Unfavorable |
Non-endocrine | ||
Amyloidosis | 3–8 | – |
Anemia | 20 | Unfavorable |
Neuromyopathy | 3 | – |
Vasculopathy | – | – |
Nephropathy | – | – |
Fever | 20 | |
Approximately 25–30 % of patients with RCC present with locally advanced or metastatic disease. Expectedly, these patients can present with symptoms secondary to metastasis to distant sites. The most common sites of metastasis include:
Lung: 50–60 % (Fig. 7.2)
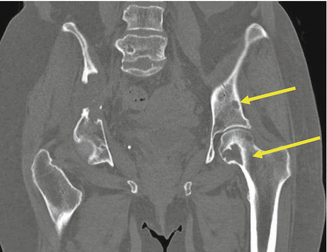
Fig. 7.3
Multiple lytic bone lesions involving the pelvis and femur from clear cell RCC. Yellow arrow shows sites of bone metastases from renal cancer
Bone: 30–40 % (Fig. 7.3)
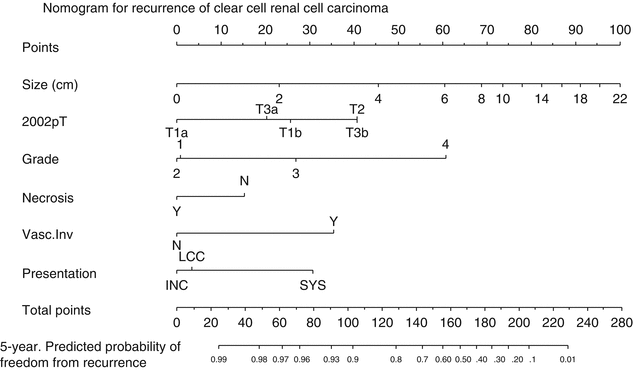
Fig. 7.4
Postoperative nomogram to predict recurrence in localized clear cell RCC (Reprinted from Sorbellini et al. [54], January 2005 with permission from Elsevier)
Liver: 30–40 %
Soft tissue: 35 %
Central nervous system: 8 %
Cutaneous: 8 %
Depending on the organ involved, patients can present with hemoptysis, pleural effusion, cough, bone pain, back pain, pathological fracture, mental status changes, and headache.
Histology also appears to influence the initial clinical presentation. Clear cell RCC has a propensity for vascular invasion and is associated with distant metastasis at an early stage as compared to the papillary tumors that tend to have locoregional invasion with lymph node spread. Due to the low potential for early vascular invasion of papillary and chromophobe cancers distant metastases typically occur later in the disease course.
7.1.2 Paraneoplastic Manifestations
Paraneoplastic syndromes are defined as a collection of symptoms and clinical signs that occur in cancer patients, remotely from the tumor location. These are the result of humoral substances produced by the cancer cells (such as calcitriol production by RCC) or benign tissues generating humoral factors in response to malignancy (such as clubbing) or via modulation of the immune system.
Approximately 20 % of patients present initially with paraneoplastic symptoms, while up to 40 % can develop some form of paraneoplastic symptoms during their disease course. After nephrectomy, the recurrence of a previous paraneoplastic syndrome should alert for possible disease progression. Because of its propensity for causing paraneoplastic symptoms, RCC has historically been called one of the “great masqueraders” of medicine [2, 3].
7.1.2.1 Hypercalcemia
This is the most common of the paraneoplastic syndromes, affecting 13–20 % of patients with RCC. Approximately 75 % of patients presenting with hypercalcemia have advanced disease, while about half have bone metastasis. Non-metastatic hypercalcemia is secondary to the elaboration of humoral peptides by RCC. These include PTHrP, IL-1, TNF, TGF, and OAF. The clinical picture can be very polymorphic. Symptoms can range from nonspecific symptoms such as asthenia, headache, lack of appetite, nausea, vomiting, constipation, polyuria, and polydipsia (due to nephrogenic diabetes insipidus) to specific clinical syndromes such as hypercalcemia or erythrocytosis or anemia. Hypercalcemia is a life-threatening condition that typically manifests with confusion, constipation, or nausea. Profound lethargy or even comatose condition has been noted. Physical findings include decreased deep tendon reflexes and an impaired level of consciousness. Patients may be dehydrated secondary to loss of renal concentrating ability and subsequent polyuria. Laboratory studies in affected patients reveal hypercalcemia, decreased levels of PTH, and 1,25-vitamin D and renal phosphate wasting. ECG findings include increased PR and QT intervals with eventual bradyarrhythmias and asystole. Treatment is mainly with repletion of volume with IV fluids and loop diuretics as needed. Bisphosphonates such as pamidronate or zoledronate are effective for long-term management. It has been suggested that the most effective way to treat the hypercalcemia is to treat the cause, with nephrectomy for localized disease and systemic therapy for metastatic RCC [2, 4].
7.1.2.2 Hypertension
Up to 40 % of patients with RCC develop hypertension as a paraneoplastic manifestation. Hypertension is typically associated with low-grade, clear cell tumors. Potential mechanisms include renin secretion, ureteral or parenchymal compression, presence of an arteriovenous fistula, and polycythemia. The sequence of events is believed to be as follows: local renal parenchymal compression and ureteral obstruction causes renin secretion, which then contributes to hypertension. Elevated serum renin levels have been found in 37 % of patients with RCC. Treatment for hypertension caused by RCC is nephrectomy; 85 % will become normotensive after such a procedure [2, 3].
7.1.2.3 Polycythemia
This is seen in 1–8 % of RCC patients, mainly mediated by erythropoietin (EPO), a glycoprotein produced by tumor cells and peritubular renal interstitial cells that promotes red blood cell production in the bone marrow. Elevated EPO levels have no prognostic significance. Patients with high EPO levels develop anemia more often than polycythemia [2, 3]. Interestingly, although two-thirds of patients with RCC have elevated EPO levels, only 8 % experience erythrocytosis.
7.1.2.4 Non-metastatic Hepatic Dysfunction (Stauffer’s Syndrome)
In 1961, Stauffer noted hepatic laboratory abnormalities in a patient with RCC with no evidence of hepatic metastases. These resolved with nephrectomy but returned with disease recurrence. Incidence of this so-called Stauffer’s syndrome is 3–20 %. Patients with this syndrome present with hepatosplenomegaly, fevers, and weight loss. It is characterized by transaminitis and abnormal hepatic synthetic function. In two-thirds of patients, nephrectomy led to resolution of Stauffer’s syndrome. One-year survival was found to be 88 % in patients whose liver enzymes normalize after nephrectomy, compared to 26 % if they remain elevated [2, 3, 5, 6].
7.1.2.5 Constitutional Symptoms
One-third of RCC cases present with constitutional symptoms like fever, weight loss, and fatigue. Twenty to thirty percent can have fever, but only 2 % have it as a sole manifestation. In a study by Tsukamoto et al., 18 of 71 patients have elevated levels of IL-6, and 78 % of those with increased levels had fever [7]. In a study by Kim et al., cachexia, defined as hypoalbuminemia, weight loss, anorexia, or malaise, predicted worse survival after controlling for well-established indicators of prognosis including TNM stage, Fuhrman grade, and ECOG-PS [8].
7.1.2.6 Other Endocrine Abnormalities
Abnormal glucose metabolism has been described in RCC. There have been several case reports of either hyperglycemia or hypoglycemia. RCC tumors have been reported to have elevated intracellular levels of insulin, glucagon, and enteroglucagon when compared to controls.
RCC accounts for 2 % of all neoplasms that are responsible for Cushing’s syndrome. This is secondary to enzymatic conversion of pro-opiomelanocortin to ACTH by the tumor. This ectopic ACTH drives cortisol secretion by the adrenal glands. Post-nephrectomy these patient are at risk for postoperative Addisonian crisis [2, 3]; thus, clinicians should be cognizant of this potential complication.
Finally, elevated serum beta-HCG levels can be found in 6 % of patients with RCC.
7.1.2.7 Non-endocrine Paraneoplastic Syndromes
Amyloidosis is seen in 3–8 % of patients with RCC. The amyloid protein found is AA. The mechanism hypothesized for AA deposition is prolonged stimulation of the immune system by either the malignancy or tumoral necrosis, leading to a rise in the levels of the acute phase reactant SAA. Initial patient complaints are weakness, weight loss, and syncope. Eventually the symptoms depend on which organ is involved.
Neuromyopathies are also described in RCC. They can be sensory or motor. Severity varies from nonspecific myalgias to a symptom complex reminiscent of amyotrophic lateral sclerosis.
7.2 Imaging (Table 7.3)
Table 7.3
Diagnostics of RCC: imaging modalities, their sensitivity and specificity
Imaging modality | Primary tumor | Perinephric extension | Lymph adenopathy | Venous thrombus/tumor | Metastasis | Staging accuracy | IVC extension | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Sn | Sp | Sn | Sp | Sn | Sp | Sn | Sp | Sn | Sp | Sn | Sp | ||
US | 91 | 99 | 21 | – | – | 100 | − | – | – | – | 54 | ||
CT | 91 | 100 | 46 | 98 | 92 | 98 | 78 | 96 | 98 | 99 | 96 | 78 | 83 |
MRI | 93 | 65 | 84 | 95 | – | – | 65 | 81 | 82 | 82 | 97 | ||
FDG-PET | 60 | 100 | – | – | 75 | 100 | – | – | 63 | 100 | 94 | – | – |
With the implementation of modern cross-sectional imaging modalities in clinical practice, the diagnosis, treatment, and surveillance of RCC have changed dramatically in the past two decades. As the incidental detection of small renal tumors has increased, this allowed earlier detection and treatment, consequently improving long-term survival rates [9, 10].
The major goals of imaging techniques are to correctly differentiate benign from malignant lesions and for early diagnosis, precise staging, and response evaluation to systemic therapy [11].
7.2.1 Ultrasound
Ultrasound (US) is often the first imaging technique used to evaluate patients with suspected RCC. Vascular flow detected by color Doppler US was reported to be strongly suggestive of conventional clear cell histology. Color Doppler US had a diagnostic accuracy similar to dynamic CT in most patients with renal solid tumors, and the color flow pattern was different among RCC subtypes. These observations suggest the use of color Doppler US as an additional tool in patients whose tumor is poorly attenuated or in those with contraindications for contrast medium and radiation [12]. When compared to CT scans, the accuracy of US to detect small renal tumors is low. The sensitivity for tumors that are <3 cm in diameter is only 67 % [13]. The deficiencies with conventional US are definitive identification of the following: complex cystic lesions, venous tumor thrombus extension, and verification of metastatic lesions. These shortcomings are due to the well-known inherent limitations of US imaging such as reliance on operator experience and on patient’s constitution.
Contrast-enhanced US (CEUS) is a rapidly evolving technique using US-specific intravenous contrast agents in the form of microbubbles. A complete concordance between CEUS and CT in the differentiation of surgical and nonsurgical complex cysts was reported [14]. The sensitivity to detect tumor thrombus can reach 100 % if it involves the intrahepatic portion of the IVC, but it drops to 68 % if it lies below the level of the insertion of the hepatic veins. Depending on the patient’s constitution, in 43.5 % of cases the IVC is not completely visualized [15]. It is the only available intraoperative imaging modality to ensure nephron-sparing surgery and to identify additional tumors. Under US guidance, minimally invasive procedures like biopsies and radiofrequency ablations can be performed [16].
Dynamic contrast-enhanced US can potentially be used in the era of antiangiogenic therapies to evaluate tumor response. An ongoing French national study will be able to define its utility in monitoring antiangiogenic therapy [17].
7.2.2 Computed Tomography (CT) Scanning
The gold standard for the diagnosis, staging, and surveillance of RCC is the CT scan [18, 19]. With multidetector-row CT (MDCT) scanners, one is able to obtain a true volume scan and ultrathin sections (<0.5 mm) with minimal time for motion artifact [20]. With the advent of triphasic (unenhanced, corticomedullary or arterial phase, and nephrographic phase) MDCT and 3D reconstruction, there is provision of accurate preoperative planning, especially for nephron-sparing surgery [21]. The degree of enhancement is a unique finding to differentiate conventional clear cell RCC from other subtypes and from angiomyolipoma [22]. Jinzaki et al. reported that clear cell RCC showed a peak attenuation value in the cortical nephrographic phase of >100 HU, whereas for other subtypes it is <100 HU [23]. The presence of homogeneous and prolonged enhancement significantly differentiates angiomyolipoma with minimal fat from RCC [24].
The staging accuracy with CT scans is 90 %. The detection of a normal adrenal gland in MDCT is associated with 100 % negative predictive value for metastasis [25]. For lymph node metastasis, the false-negative rate is 10 %, and false-positive rate ranges from 3 to 43 % [26, 27]. For M staging, there is an excellent agreement between MDCT and surgical pathology [27]. With the MDCT, tumor thrombus is accurately identified and localized.
Tumor response to antiangiogenic therapy can also be assessed with CT scanning. The application of RECIST criteria is limited in tumors with irregularity and diffuse invasion. So volumetric mean tumor attenuation in contrast-enhanced MDCT has been proposed as an alternative potential response criterion.
7.2.3 Magnetic Resonance Imaging (MRI)
MRI is the imaging modality of choice in patients with contrast allergy and functional renal impairment or who are pregnant. It is mainly used as a complementary problem-solving tool in selected cases of undefined renal lesions and suspected perinephric tumor spread or recurrence. The advantages of MRI include: absence of radiation, lack of need for standard iodinated contrast medium, and its high inherent contrast among different soft tissues [16]. Disadvantages are longer examination times, higher cost, and inferior capacity to detect lung metastasis. In patients with renal insufficiency, the MRI contrast medium gadolinium has been associated with nephrogenic systemic fibrosis.
In a study by Pedrosa et al., the overall sensitivity and specificity of MRI to predict the histologic subtype were 92 and 83 % for clear cell and 80 and 94 % for papillary RCC, respectively [28]. MRI along with CT scans has difficulty in correctly identifying perinephric tumor invasion, distinguishing inflammation from tumor infiltration, and insensitivity in differentiating small collateral blood vessels from tumor extension in the lymphatics [29]. The sensitivity and specificity for detecting metastatic lymphadenopathy are low. It is highly sensitive and specific for detection of bone metastasis [30]. It is more sensitive than CT for detection of brain metastasis. MRI is a reliable method for evaluation of tumor thrombus. The accuracy is ranging from 65 to 100 % [16].
In regard to response evaluation to antiangiogenic therapy, it is still restricted to clinical trials because of poor standardization, methodologic challenges, limited sensitivity, and concerns related to potential harmful effects of MRI contrast agents.
7.2.4 FDG- PET
The increased background activity of healthy renal tissue and normal FDG excretion in urine can make visualization of primary renal cancers by PET difficult. 2-Deoxy-2-[18F]-fluoro-D-glucose (FDG) thus far has not offered any advantage over a standard imaging modality such as MDCT. In a retrospective review [31], the sensitivity and specificity of PET for detection of primary RCC were 60 % and 100 %, respectively, and with CT scan, these were 91.7 % and 100 %, respectively. It is also less sensitive than CT in the detection of metastasis to retroperitoneal lymph nodes and/or renal bed recurrence (75 vs. 92.6 %), lung metastases (75 vs. 91.1 %), and bony metastases (77.3 vs. 93.8 % of CT + bone scan). By using PET with an iodine-124-labelled antibody chimeric G250 (124I-cG250) against carbonic anhydrase-IX (“immuno-PET”) for clear cell RCC, sensitivity was 94 % and specificity was 100 % [32]. Other markers under investigation are 18F-fluoromisonidazole (FMISO), a noninvasive tumor marker of tissue hypoxia, and 18F-fluorothymidine, a tracer that mirrors cellular proliferation.
FDG-PET/CT has the advantage to detect the metabolic activity of local recurrence that is not influenced by factors that jeopardize diagnosis of local recurrence with CT, such as migration of the adjacent normal organs into the renal fossa, postoperative scarring, and artifacts from surgical clips [33]. FDG-PET/CT can examine the whole body in one procedure without contrast agents. Park et al. demonstrated that, for the surveillance of high-risk RCC, FDG-PET/CT had results as good as conventional methods and were not influenced by the Fuhrman grade or the histological subtype. FDG-PET/CT is 89.5 % sensitive, 83.3 % specific, and 85.7 % accurate in detection of recurrence or metastasis.
7.3 Staging
Tumor stage, which reflects the anatomic spread and involvement by disease, is recognized as the most important prognostic factor for the clinical behavior and outcome of RCC. The first formal staging system proposed by Flocks and Kadesky in 1958 was based on the physical characteristics of the tumor and the location of tumor spread.
Currently the staging system that is followed is the tumor-node-metastasis (TNM) system. It was most recently revised in 2010 and is supported by both the American Joint Committee on Cancer (AJCC) and the International Union for Cancer Control (UICC). This is a dynamic staging method that changes continually on the basis of new evidence from clinical studies. It is based on data from large multicenter studies with a fairly good level of evidence.
The first TNM staging system was developed in 1978. Tumors are characterized on the basis of the degree of local extension of the tumor at the primary site (T), the involvement of regional lymph nodes (N), and the presence or absence of distant metastases (M). The classification may be clinical (cTNM) or histopathological (pTNM). Regional lymph nodes for RCC are defined as the hilar, abdominal para-aortic, and paracaval nodes [34, 35]. Refer to Table 7.4 for a full description of the TNM staging system for RCC.
Table 7.4




Revised 2010 AJCC TNM staging system
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree