Chapter 31 Therapeutic Vaccines
IMMUNOLOGIC RATIONALE
Increasing evidence has demonstrated that adaptive immune responses are critical for control of viral replication. A therapeutic vaccine aims to enhance HIV-1-specific immunity and to improve this immune control. CD8+ T-lymphocytes exert antiviral cytolytic activity as well as other effector functions, and CD4+ T-lymphocytes provide immunologic ‘help’ to B cells and CD8+ T-lymphocytes.1–3 The importance of CD8+ T-lymphocytes has been directly demonstrated by experimental depletion of CD8+ T-lymphocytes in SIV-infected rhesus macaques by monoclonal antibody administration.4,5 Moreover, in humans with acute HIV-1 infection, potent CD8+ T-lymphocyte responses were temporally correlated with initial control of HIV-1 viremia.6,7 Long-term nonprogressors (LTNPs) have also been shown to have vigorous HIV-1-specific CD8+ and CD4+ T-lymphocyte responses that were associated with preservation of CD4+ T-lymphocyte counts and durable control of viral replication without the use of ART.1,8,9 T-lymphocytes from LTNPs retain a high proliferative capacity and exhibit high levels of perforinexpression, suggesting the potential importance of these immune functions.10
Patients who initiate ART during acute HIV-1 infection typically have preserved HIV-1-specific CD41 T-lymphocyte responses as well as detectable CD8+ T-lymphocyte responses.2,11–13 In an observational study, supervised treatment interruptions (STIs) of HIV-1-infected individuals treated with ART during acute infection resulted in viral rebound, which in turn stimulated expansion of HIV-1-specific CD4+ and CD8+ T-lymphocyte responses and led to transiently improved virologic control during subsequent STIs.13 Although the durability of this immune control was limited,14 this study showed that immune-based interventions can exert detectable effects on viral replication. STI studies in chronically HIV-1-infected individuals, however, have failed to demonstrate improved control of viral replication.15 In addition, a recent study has highlighted potential safety concerns associated with performing STIs in chronically HIV-1-infected individuals.16 These data suggest that it may be substantially more challenging to design therapeutic vaccines for the majority of patients who are treated during chronic HIV-1 infection as compared with individuals who begin ART during acute HIV-1 infection.
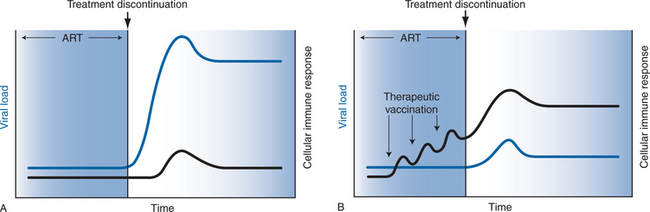
The immunologic rationale of therapeutic vaccination is illustrated in Figure 31-1. As shown in Figure 31-1A, HIV-1-infected subjects treated with effective ART typically exhibit low levels of HIV-1-specific humoral and cellular immune responses and undetectable viral RNA levels. Following discontinuation of ART, HIV-1 RNA levels predictably rebound within several weeks to pretreatment ‘setpoint’ levels. As shown in Figure 31-1B, therapeutic vaccination in the context of suppressive ART may enhance virus-specific immune responses, which could improve immune control of viral replication following discontinuation of ART.
Although there is currently no evidence that HIV-1 therapeutic vaccines will afford any clinical benefit, a recent study has demonstrated the clinical utility of a therapeutic vaccine for a different virus. A live, attenuated varicella-zoster virus (VZV) vaccine has been shown to reduce the incidence and severity of illness from clinical herpes zoster as well as the incidence of postherpetic neuralgia, demonstrating that therapeutic vaccination for this persistent viral infection can result in clinical benefits.17 Although the mechanism of action of the VZV vaccine remains to be established, it presumably involves enhancement of VZV-specific immunity. However, VZV causes latent infection without active viral replication, whereas HIV-1 causes chronic infection with persistent viral replication, and thus it will likely prove substantially more challenging to produce an HIV-1 therapeutic vaccine than a VZV therapeutic vaccine.
NONHUMAN PRIMATE STUDIES
A proof-of-concept study in the macaque model utilized a recombinant poxvirus vaccine expressing SIV structural genes (NYVAC-SIV). In rhesus macaques treated with ART following primary SIV infection, vaccination with NYVAC-SIV augmented SIV-specific CD4+ and CD8+ T-lymphocyte responses. Following discontinuation of ART, all animals exhibited viral rebound, but SIV replication was spontaneously controlled in six of eight vaccinated animals and in four of seven unvaccinated controls.18 Although the observed virologic control following treatment discontinuation likely reflected the fact that ART was initiated during acute infection, this study nevertheless demonstrated that virus-specific immune responses could be enhanced by therapeutic vaccination. In a second study from this same group, NYVAC-SIV vaccines expressing gag-pol-env and rev-tat-nef were administered with interleukin-2 (IL-2) as an adjuvant to chronically SIV-infected macaques on ART.19 Following treatment discontinuation, viral setpoint was significantly lower in vaccinated macaques than in control animals, and this difference persisted for at least 4 months. Viral containment in this study was also inversely correlated with vaccine-induced virus-specific CD41 and CD8+ T-cell responses.
Therapeutic vaccine strategies utilizing plasmid DNA vaccines have focused on novel delivery approaches, given the relatively low immunogenicity of DNA vaccines in nonhuman primates and humans as compared with small animal models. One strategy involves transfecting DCs with plasmid DNA vaccines and then administering these DCs to macaques.20 A topical DNA vaccine has also been developed and was shown to enhance SIV-specific cellular immune responses in chronically SIV-infected macaques on ART.21 Following withdrawal of ART, vaccinated animals exhibited improved control of viral rebound as compared with unvaccinated controls. Another study demonstrated that autologous DCs pulsed with inactivated SIV enhanced virus-specific cellular immune responses and resulted in a substantial reduction in viral RNA levels in vaccinated animals as compared with unvaccinated controls.22
CLINICAL STUDIES
As shown in Table 31-1, studies of therapeutic HIV-1 vaccines in humans have explored a variety of different vaccine modalities, including recombinant protein subunits, inactivated virus particles, virus-like particles, plasmid DNA vaccines, recombinant poxvirus vector-based vaccines and DC-based vaccines.23–41 These studies have demonstrated the safety and immunogenicity of several vaccine approaches, but none of these studies have convincingly demonstrated clinically meaningful endpoints. In HIV-1-infected individuals receiving ART, vaccination with the attenuated poxvirus vectors ALVAC vCP1452 and modified vaccinia Ankara (MVA) proved safe and immunogenic.34–3638 The magnitude of vaccine-enhanced immune responses elicited by both of these vectors, however, was modest. Similarly, an inactivated, envelope-depleted HIV-1 preparation (Remune) was shown to be safe and immunogenic28, but a large phase III trial failed to show any significant effect on HIV-1 viral RNA levels or progression-free survival.27 The lack of a beneficial effect in this study may have reflected incomplete suppression of viral replication with ART prior to vaccination as well as the inability of this vaccine to elicit potent virus-specific CD8+ T-lymphocyte responses.
Table 31-1 Results of Recent HIV-1 Therapeutic Vaccine Clinical Studies
| Vaccine | Patient Characteristics | Results |
|---|---|---|
| Protein Subunits | ||
| rgp160 (Kundu et al23) | Chronic HIV-1 infection, CD4 >400 cells/μL, not on ART; n = 21 vaccinees, five controls | Increased CD4+ and CD8+ T-cell responses |
| rgp160 (Goebel et al24) | Chronic HIV-1 infection; CD4 >200 cells/μL; some patients on ART; n= 103 vaccinees, 105 controls | Increased lymphoproliferative responses; no differences in CD4 counts, HIV-1 RNA levels or clinical outcomes |
| rgp160 (Birx et al25) | Chronic HIV-1 infection; CD4 >400 cells/μL; not on ART; n =302 vaccinees, 306 controls | Increased humoral and lymphoproliferative responses; no differences in CD4 counts, HIV-1 RNA levels or clinical outcomes |
| rgp120 (Schooley et al26) | Chronic HIV-1 infection; CD4 >50 cells/μL; some patients on ART; n= 200 vaccinees, 98 controls | Increased lymphoproliferative responses |
| Inactivated Virus | ||
| Remune (Kahn et al27) | Chronic HIV-1 infection; CD4 300 cells/μL; some patients on ART; n = 1262 vaccinees, 1265 controls | Increased lymphoproliferative responses; no differences in CD4 percents, HIV-1 RNA levels or clinical outcomes |
| Remune (Robbins et al28) | Chronic HIV-1 infection; CD4 >250 cells/μL; HIV-1 RNA levels <500 copies/mL; on ART; n = 5 vaccinees, five controls | Increased lymphoproliferative responses |
| Virus-Like Particle | ||
| p24 virus-like particle (Kelleher et al29) | Chronic HIV-1 infection; CD4 >400 cells/μL; not on ART at enrollment; n = 41 vaccinees, 20 controls | No changes in humoral or lymphoproliferative responses; no differences in HIV-1 RNA levels |
| DNA | ||
| DNA-nef/rev/tat (Calarota et al30) | Chronic HIV-1 infection; some subjects on ART; n = 9 vaccinees | Increased CD8+ T-cell responses |
| DNA-env/rev (MacGregor et al31) | Chronic HIV-1 infection; CD4 >500 cells/μL, not on ART; n = 15 vaccinees | Increased humoral, lymphoproliferative responses; no differences in CD4 counts or HIV-1 RNA levels |
| DNA-HIV A (Dorrell et al32) | Chronic HIV-1 infection; CD4 >300 cells/μL; HIV-1 RNA levels <400 copies/mL; on ART; n =10 vaccinees | Increased CD4+ and CD8+ T-cell responses in minority of subjects |
| DNA-nef/MultiHIV B (Blazevic et al33) | Chronic HIV-1 infection; on ART; n = 27 vaccinees | No humoral responses; increased CD4+ and CD8+ T-cell responses |
| Poxvirus | ||
| MVA-nef (Cosma et al34) | Chronic HIV-1 infection; CD4 >400 cells/μL; on ART; n = 10 vaccinees | Increased CD4+ T-cell responses |
| MVA-nef (Harrer et al35) | Chronic HIV-1 infection; CD4 >400 cells/μL; on ART; n = 14 vaccinees | Increased CD4+ and CD8+ T-cell responses |
| MVA-HIV A (Dorrell et al36) | Chronic HIV-1 infection; CD4 >300 cells/μL; HIV-1 RNA levels <50 copies/mL; on ART; n = 16 vaccinees two controls | Increased CD4+ and CD8+ T-cell responses |
| Poxvirus + Protein | ||
| ALVAC vCP1452 + rgp160 (Markowitz et al37; Jin et al38) | ART initiated during acute or early HIV-1 infection; HIV-1 RNA levels <50 copies/mL; n = 11 vaccinees, five controls | Increased humoral, lymphoproliferative and CD8+ T-cell responses; no differences in HIV-1 RNA levels after discontinuation of ART |
| Poxvirus + Inactivated Virus | ||
| ALVAC vCP1452 ± Remune (Kinloch-de Loes et al39) | ART initiated during acute HIV-1 infection; HIV-1 RNA levels <50 copies/mL; n = 52 vaccinees, 27 controls | Increased CD4+ and CD8+ T-cell responses; no differences in CD4 counts or HIV-1 RNA levels after discontinuation of ART |
| Poxvirus + Lipopeptides + IL-2 | ||
| ALVAC vCP1433 + Lipo-6T + IL-2 (Levy et al40) | Chronic HIV-1-infection; CD4 >350 cells/μL; on ART; n = 34 vaccinees, 37 controls | Increased lymphoproliferative, CD4+ and CD8+ T-cell responses; 24% vaccinees vs 5% controls had lower HIV-1 RNA levels after discontinuation of ART |
| Dendritic Cells + Inactivated HIV-1 | ||
| Autologous dendritic cells loaded with inactivated virus (Garcia et al41) | Chronic HIV-1 infection; CD4 >500 cells/μL; on ART; RNA levels <20 copies/mL; n = 12 vaccinees, six controls | No change in humoral or T-cell responses; decreased HIV-1 RNA levels in four subjects |
| Autologous dendritic cells loaded with inactivated virus (Lu et al42) | Chronic HIV-1 infection; CD4 >300 cells/μL; not on ART; n = 18 vaccinees | Increased CD4+ and CD8+ T-cell responses; reduction in HIV-1 RNA levels from baseline values in eight subjects |
A randomized, double-blinded, placebo-controlled study has also recently evaluated the impact of therapeutic vaccination with ALVAC vCP1452 and/or Remune in 79 HIV-1-infected subjects treated with ART during acute infection.39 These vaccines led to detectable increases of HIV-1-specific CD4+ and CD8+ T-cell responses, but they failed to afford any improvement in virologic control 24 weeks after discontinuation of ART. This was a disappointing result, since subjects treated during acute HIV-1 infection theoretically should be the most amenable to immune-based interventional strategies as a result of their relatively preserved immune systems. There was also no significant correlation between gag-specific CD4+ and CD8+ T-lymphocyte responses and viral RNA levels following discontinuation of ART in this study, suggesting that the correlates of immunity are more complex than simply the magnitude of these immune responses.
Another study has recently investigated therapeutic vaccination with ALVAC vCP1433 and HIV-1 lipopeptides (Lipo6T) together with IL-2 as an adjuvant in chronically HIV-1-infected subjects on ART. In this study, a higher percentage of vaccinees developed significant HIV-1 p24 proliferative responses and CD4+ T-lymphocyte responses as compared with controls. The breadth and magnitude of HIV-1-specific IFN-γ-producing CD8+ T-lymphocyte responses also improved in the vaccine group as compared with the control group. After treatment discontinuation, 24% of vaccinees exhibited a statistically significant reduced viral setpoint as compared with only 5% of controls, and virologic control correlated with vaccine-elicited cellular immune responses.40
Another novel therapeutic vaccine approach involves the use of antigen-loaded autologous DCs. In one study, autologous DCs were pulsed with virus obtained during an initial interruption of ART and were then utilized as a therapeutic vaccine after ART was re-initiated. Following a second interruption of ART, there was a significant lengthening in mean doubling time during the viral rebound.41 In a second study, 18 chronically HIV-1-infected individuals who were not receiving ART were treated with monocyte-derived DCs loaded with autologous inactivated HIV-1. HIV-1 RNA levels in vaccinated individuals decreased by 80% over the first 112 days, and eight subjects had virologic suppression of over 90% for at least 1 year.42 Moreover, virologic suppression appeared to correlate with HIV-1-specific CD4+ and CD8+ T-lymphocyte responses. These results suggest that certain therapeutic vaccines can indeed exert a degree of virologic control, but these studies involved only a limited number of subjects and the clinical relevance of these observations remains unclear.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree