The Roles of Ultrasound and Magnetic Resonance Imaging in the Evaluation of the Breast
Imaging the breast has evolved from mammography alone to mammography with adjunctive modalities that primarily include ultrasound and magnetic resonance imaging (MRI). Until recent years, ultrasound and MRI have functioned as problem-solving tools and have been used to help to diagnose a mammographically and/or clinically detected abnormality. As these modalities have improved technologically and vast clinical experience has been acquired, so have their roles expanded from diagnostic tools to potential screening tools as well.
Ultrasound has many uses that relate to the evaluation of breast masses. Sonographic guidance is used frequently for the direction of percutaneous breast biopsies. MRI was initially used for the evaluation of implants, but it now has several roles that relate to the evaluation of carcinomas. With a dedicated breast coil and contrast enhancement, MRI has been of great value in identifying the extent of carcinoma and in helping to better plan patient management.
In this chapter, the discussion will not focus on the technical aspects of ultrasound or MRI. Instead, the focus will be on the roles of these ancillary modalities in complimenting mammography and the information that can be provided to enhance the clinical knowledge about the patient.
The Role of Breast Ultrasound
The role of breast ultrasound has expanded considerably as the technique and resolution of the equipment have improved. With high-frequency linear array transducers and higher resolution, smaller lesions and greater detail are visualized. The initial primary role of ultrasound was the differentiation of solid versus cystic masses (1,2,3,4). Sonography is ideal for this role and is important in planning the management of the patient. The roles of ultrasound are shown in Table 17.1.
Of paramount importance in incorporating ultrasound in a breast-imaging program is the use of high-quality equipment and proper technique. High-frequency linear array transducers are typically used. Current transducers have frequency ranges of 15-18 MHz, 13-8 MHz, 12-5 MHz and 10-5 MHz (5). Setting the focal zone to the proper depth, at the level of the area of interest, optimizes the analysis of the lesion of interest. Setting the power and time-gain compensation at the proper level is extremely important so that the analysis of cystic versus solid masses is accurate. If the gain is set too low, a hypoechoic solid mass may be interpreted as anechoic and thought to be a cyst when in fact it is solid. Scanning may be performed in a longitudinal and transverse pattern or in a radial and antiradial orientation (5).
The ACR BI-RADS® Lexicon for Breast Imaging (6) also has descriptions for ultrasound reporting. In the BI-RADS® for ultrasound, the following are described: background echotexture, mass features, calcifications, special cases, and vascularity. Masses are described (6) according to their shape, orientation, margins, lesion boundary, echo pattern, posterior acoustic enhancement features, and
effect on surrounding tissue. The background echotexture may be homogeneous-fat, homogeneous-fibroglandular, or heterogeneous.
effect on surrounding tissue. The background echotexture may be homogeneous-fat, homogeneous-fibroglandular, or heterogeneous.
TABLE 17.1 The Roles of Ultrasound | |
|---|---|
|
Mass shapes may be oval, round, or irregular, and their orientation is parallel or not parallel to the chest wall. The margination of masses may be described as circumscribed or not circumscribed (indistinct, angular, microlobulated, or spiculated). The boundary of the lesion may be abrupt, or the mass may be surrounded by an echogenic halo. These findings parallel mammographic findings in terms of the likelihood of benign versus malignant etiologies.
The echogenicity of masses may be described as anechoic, hyperechoic, hypoechoic, or isoechoic relative to the surrounding parenchyma, or they may be complex. Posterior acoustic features may be described as shadowing, enhancement, or no effect. Observation of the effect on surrounding tissue may include the following: ductal extension, changes in Cooper’s ligaments, edema, architectural distortion, skin thickening, or skin retraction (6).
Assessment of Isodense Circumscribed Masses: Cystic Versus Solid
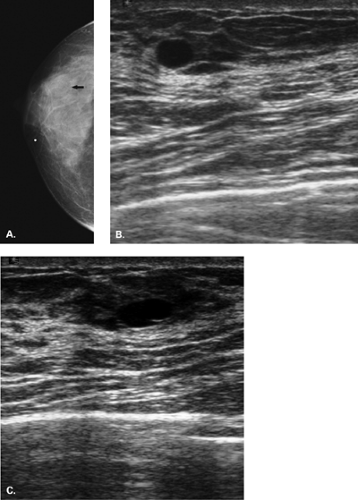
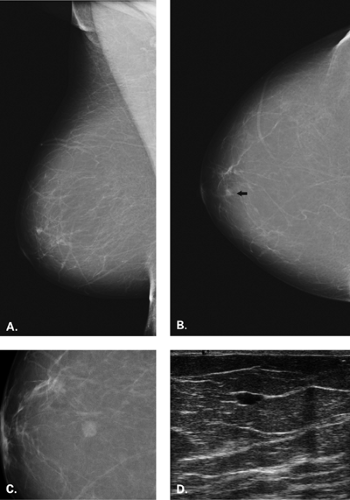
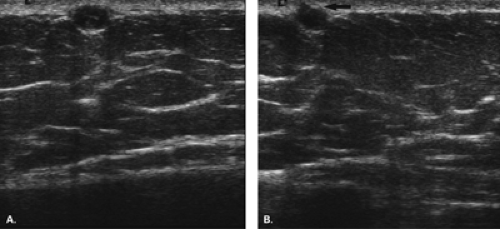
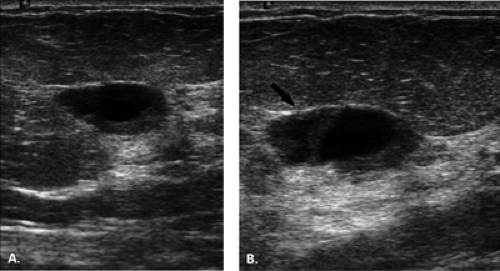
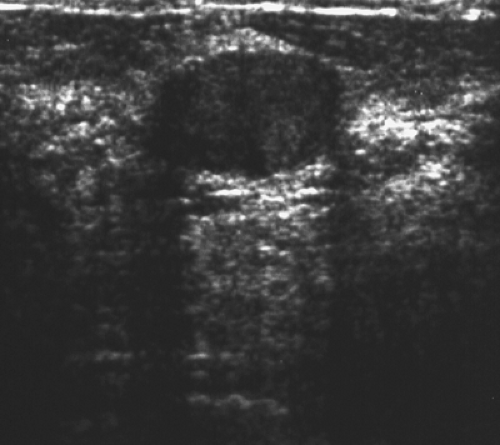
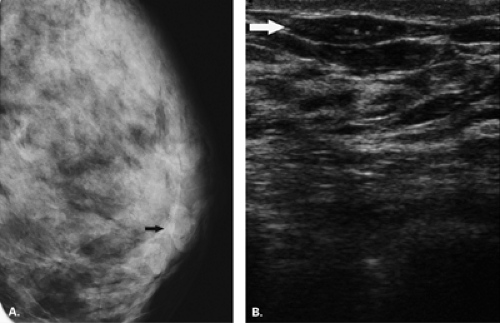
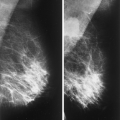
The diagnosis of a simple cyst with ultrasound is very accurate and has been reported to range from 96% to 100% (1,2,3,4,7). Careful scanning technique that includes proper gain, transducer frequency, and resolution of the equipment is necessary in order to achieve this highly reliable differentiation of solid versus cystic lesions. In addition, the criteria for a simple cyst must be adhered to in order to confirm that a lesion is clearly benign. The criteria for a simple cyst are as follows: smooth thin walls, no internal echoes (anechoic), and posterior acoustic enhancement (Figs. 17.1,17.2,17.3). With compound imaging where the scan
plane is multidirectional, posterior acoustic enhancement may not be observed for some small cysts.
plane is multidirectional, posterior acoustic enhancement may not be observed for some small cysts.
Importantly, the presence of acoustic enhancement behind a round or oval mass does not necessarily indicate that it is a cyst. Solid homogeneous masses, including breast cancers, may have acoustic enhancement as well. All the above-noted criteria must be met to call a mass a simple cyst. Thin septae may be present in a cyst and do not require biopsy. However, thick septae, a thick wall, or an intracystic solid component raises the concern for malignancy, and these findings do require tissue sampling (1,8).
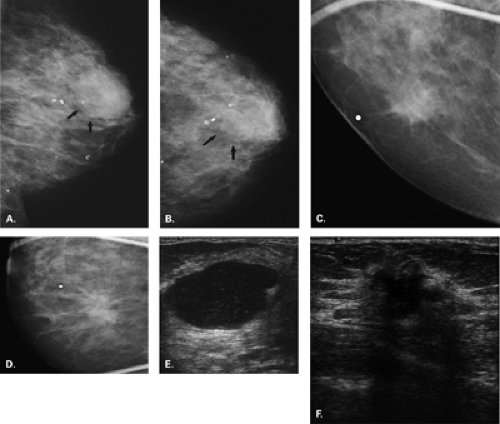
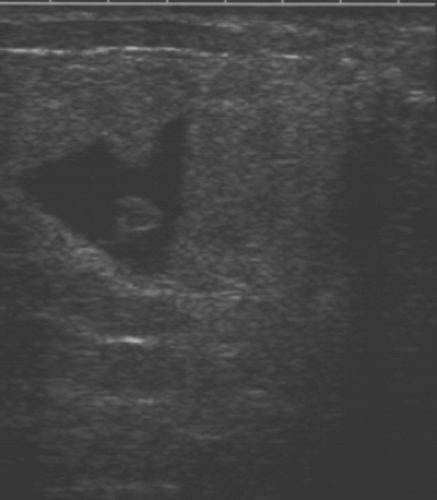
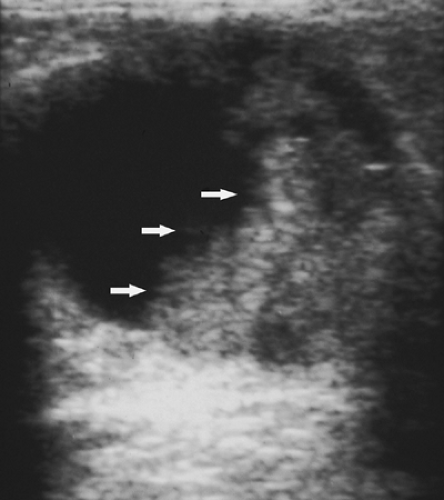
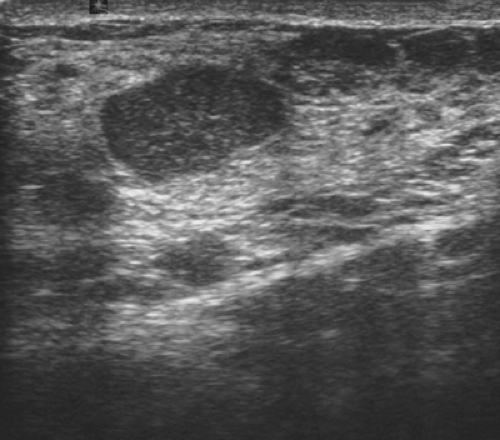
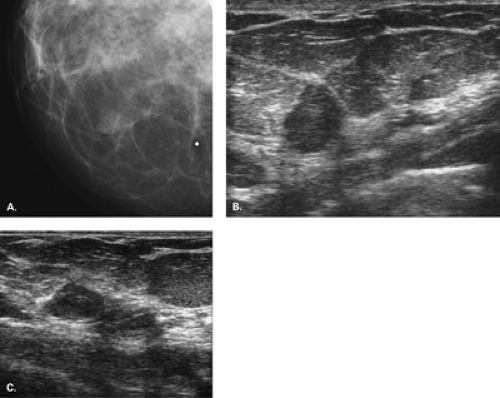
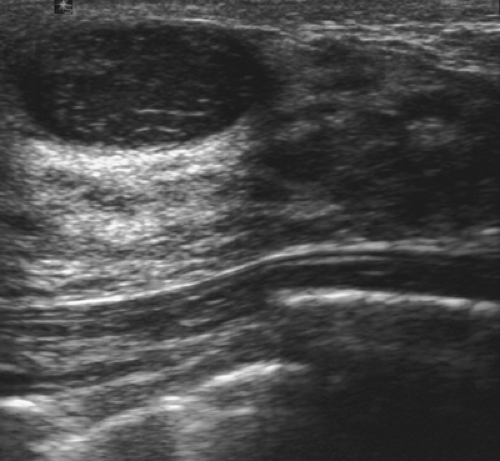
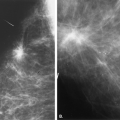
Complicated cysts are those that contain low-level echoes. This may occur secondary to hemorrhage or inflammation or because the fluid is very thick (Fig. 17.4). Occasionally a very hypoechoic solid mass may have the appearance of a complicated cyst. Aspiration is usually performed when this finding is observed, especially if the mass is new, larger, or solitary. In a study of 150 cystic lesions, Berg et al. (9) found that none of the 38 complicated cysts that were aspirated proved to be malignant. Sebaceous cysts most often have the appearance of complicated cysts (Fig. 17.5), and because of their superficial location, their etiology is evident. Also, with sebaceous cysts, a thin track between the cyst and the skin surface is often visible.
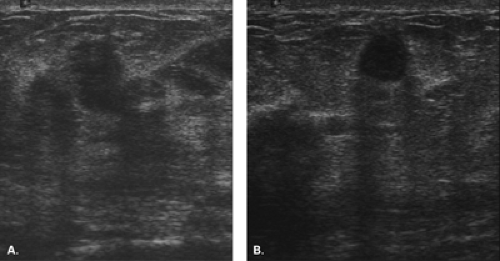
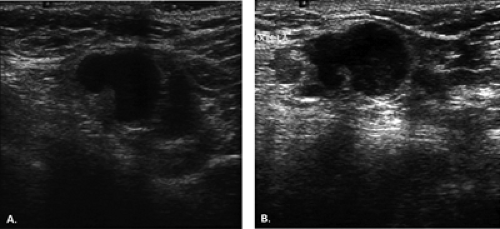
Complex masses are those that contain both cystic and solid components. A thick rind around a cystic center, polypoid solid projections within a cystic mass, or a mass that has both fluid and solid components is complex. Such masses may represent necrotic tumors or intracystic benign or malignant tumors, but they also may represent several benign lesions. A cyst with marked inflammation or hemorrhage and clot may have this appearance. Similarly,
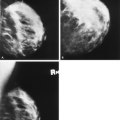
abscesses and hematomas usually appear as complex masses (Figs. 17.6 and 17.7). The management of a complex mass usually requires biopsy unless the finding is clearly a hematoma. Berg et al. (9) found that 7/23 complex masses with thick walls or thick septae and 4/18 lesions with an intracystic solid component proved to be malignant. Intracystic papillary carcinomas and necrotic high-grade malignancies may appear as complex masses on sonography (Figs. 17.8,17.9,17.10). Sampling of the solid portion of the mass is important, rather than aspiration of the fluid, so ultrasound has an important role in guiding the biopsy. Either percutaneous biopsy directed at the solid component of the mass or excision of the entire lesion is usually performed for diagnosis.
abscesses and hematomas usually appear as complex masses (Figs. 17.6 and 17.7). The management of a complex mass usually requires biopsy unless the finding is clearly a hematoma. Berg et al. (9) found that 7/23 complex masses with thick walls or thick septae and 4/18 lesions with an intracystic solid component proved to be malignant. Intracystic papillary carcinomas and necrotic high-grade malignancies may appear as complex masses on sonography (Figs. 17.8,17.9,17.10). Sampling of the solid portion of the mass is important, rather than aspiration of the fluid, so ultrasound has an important role in guiding the biopsy. Either percutaneous biopsy directed at the solid component of the mass or excision of the entire lesion is usually performed for diagnosis.
A solid mass on ultrasound is analyzed based on its contour, margination, orientation, and echogenicity. Although ultrasound cannot definitely diagnose a solid lesion as benign or malignant, there are criteria that are helpful in predicting a benign versus a malignant etiology. These features are based on the nature of benign masses, such as fibroadenomas, which are homogeneous lesions that have an encapsulated, very-well-defined margin (Figs. 17.11,17.12,17.13,17.14). Stavros et al. (10,11) described the criteria for solid benign versus malignant masses. The features of benign solid masses are listed in Table 17.2.
The sonographic features of fibroadenomas have been described in early papers (12,13) as hypoechoic, oval masses. Fibroadenomas are very well defined with a thin margin, and occasionally a pseudocapsule is evident. The lesions are usually elongated and wider than tall in orientation. Sometimes thin hyperechoic septae are seen with fibroadenomas. Most fibroadenomas have no effect on sound transmission, but some may enhance or shadow. When shadowing is identified, or when the orientation is not elongated or the borders are not well defined, biopsy is performed. A lesion that may have a similar appearance to a fibroadenoma is a phylloides tumor. On mammography, a large circumscribed mass is seen; on ultrasound, the mass is typically circumscribed and hypoechoic. The epithelial-lined clefts inside the mass may appear as small cystic spaces within the solid lesion on ultrasound (Fig. 17.15) (14).
TABLE 17.2 Ultrasound Features of Benign Solid Masses | |
|---|---|
|
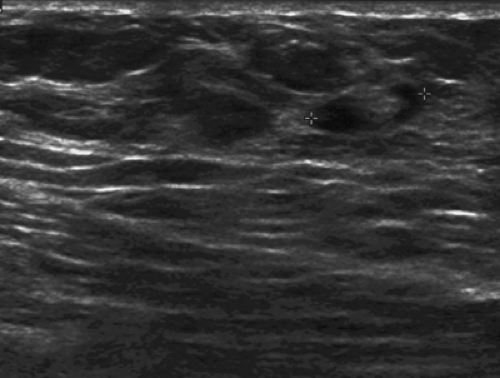
Lesions that are hyperechoic on ultrasound are typically those that contain fat. Hyperechoic masses include the following: fat necrosis (Figs. 17.16 and 17.17), lipomas, hamartomas, angiomas (Fig. 17.18), and focal fibrosis. The appearance of fat necrosis ranges from anechoic masses to complex masses with mural nodules to solid masses of varying echogenicity (15). Lymph nodes also are hyperechoic in part, depending on the amount of fatty replacement. Normal nodes are elliptical and have a smooth cortex that is hypoechoic and a bright hyperechoic center that is the fatty hilum (16) (Fig. 17.19). Abnormal nodes have a thickened cortex and an indistinct margin, and they may have an increased anteroposterior (AP) diameter with obliteration of the fatty hilum (Fig. 17.20).
Based on the Stavros et al. (10) criteria, the likelihood of a solid mass with benign characteristics actually being benign is greater than 98%. Therefore, for a nonpalpable circumscribed mass on mammography that has benign sonographic features, early follow-up may be performed. However, for new palpable or enlarging solid masses, even with benign features, biopsy is usually performed for confirmation.
Malignancies tend to be more indistinct in margination and more firm, so their AP diameter is often greater than their transverse diameter. The features of malignant masses on ultrasound are listed in Table 17.3. Malignant masses may be markedly hypoechoic, irregular (17) shadowing lesions (Figs. 17.21,17.22,17.23,17.24). Because of the dense stromal reaction, the posterior acoustic shadowing with cancers may be intense. Some cancers have branching
linear extensions around their borders, which may indicate intraductal extension of tumor. Angular or microlobulated margins are also suspicious features for malignancy. A vertical orientation (taller than wide) is an ominous sign and has a high positive predictive value of being malignant (18,19). The highest positive predictive values for malignancy on the ultrasound of masses have been found to be the following findings: spiculation (87%), a thick hyperechoic halo (74%), and a taller-than-wide orientation (74%). The lowest predictive values of the malignant signs were associated with microlobulations and ductal extension (11).
linear extensions around their borders, which may indicate intraductal extension of tumor. Angular or microlobulated margins are also suspicious features for malignancy. A vertical orientation (taller than wide) is an ominous sign and has a high positive predictive value of being malignant (18,19). The highest positive predictive values for malignancy on the ultrasound of masses have been found to be the following findings: spiculation (87%), a thick hyperechoic halo (74%), and a taller-than-wide orientation (74%). The lowest predictive values of the malignant signs were associated with microlobulations and ductal extension (11).
TABLE 17.3 Sonographic Features of Malignant Solid Masses | |
|---|---|
|
Some cancers, particularly medullary or mucinous carcinomas, have a pushing edge and are homogeneous on ultrasound. These tumors may look round and smooth and be very hypoechoic, and because of the homogeneity of the mass, they may be associated with posterior acoustic enhancement. One must use great care in order not to misinterpret a medullary or a mucinous malignancy as a cyst. Also, some very-high-grade cancers or necrotic cancers may have this same appearance. Often in these cases, though, the orientation of the lesion is vertical rather than elongated or horizontal, as is seen in benign lesions.
A variety of benign conditions may have sonographic features that are indeterminate or frankly suspicious for malignancy. Prominent shadowing is associated with dense stromal areas that may be seen in scars, focal fibrosis, hyalinized fibroadenomas, and diabetic fibrous mastopathy (Fig. 17.25). Hypoechoic masses that are not completely circumscribed or that are inhomogeneous are
seen in fibrocystic conditions at times. Radial scars (Fig. 17.26) and papillomas may also appear as solid masses that are not clearly benign.
seen in fibrocystic conditions at times. Radial scars (Fig. 17.26) and papillomas may also appear as solid masses that are not clearly benign.
An algorithm for the management of solid breast masses based on their sonographic features has been described by Stavros et al. (11). Based on this algorithm, one must first assess the lesion for any suspicious features. If a single suspicious feature is present, biopsy should be performed. If there are no suspicious features, one must search for benign characteristics. If there are no benign findings, classify the lesion as BI-RADS® 4A, suspicious, and perform a biopsy. If all the features are benign, the lesion is likely benign, and one can perform an early follow-up ultrasound.
Ultrasound in the Evaluation of the Palpable Mass
Breast ultrasound plays a critical role in the assessment of the patient with a palpable lump. For patients with a lump that is visible on mammography as a circumscribed mass, ultrasound differentiates cystic from solid etiologies. In the past, before percutaneous breast biopsy was widely used, a suspicious mass on mammography was not evaluated with ultrasound (4). However, both nonpalpable and palpable suspicious masses are often now imaged sonographically to determine if they are visible and if they can be biopsied with ultrasound guidance.
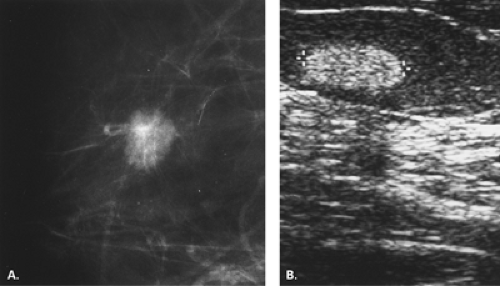
In the patient with a palpable mass and with vague, nonspecific mammographic findings or a negative mammogram, ultrasound is the next step in evaluation. Ultrasound may demonstrate a cancer or a benign mass that is not evident on mammography (Fig. 17.27). Various studies have shown the value of ultrasound in demonstrating palpable cancers that are mammographically occult (4,20,21,22,23,24).
In addition, a negative ultrasound is an important factor in determining the patient’s management. A negative ultrasound and negative mammogram in the clinical situation of a palpable mass are associated with a risk of malignancy of <2%. Depending on the level of suspicion on clinical exam, clinical follow-up rather than biopsy may be performed when imaging is completely negative (25).
Ultrasound in Young or Pregnant Patients
In women younger than age thirty with a palpable mass, the most likely diagnosis is a fibroadenoma, with cysts and cancers being less likely. The workup of the patient is affected by the very low frequency of cancers is this age group and by the somewhat increased radiosensitivity of the breasts. Typically, mammography is not performed first, unless the patient is at high risk for breast cancer.
Most breast imagers (4,18,26,27) begin with ultrasound in the symptomatic young patient (Fig. 17.28). The exception to this is the very-high-risk patient (BRCA1 or BRCA2 carrier, history of treated Hodgkin disease, strong premenopausal family history, personal history of breast cancer). In the very-high-risk patient, bilateral mammography is performed first. A pitfall in this age group is the assumption that the patient has very dense breasts and that mammography is not helpful or will not detect cancer. Often in young women with breast cancer, a delay in diagnosis occurs for this reason (28).
In the usual-risk patient, ultrasound is performed to determine if the palpable mass is solid, cystic, or not seen. If the lesion is a cyst, no further workup is needed. If the lesion is solid and has the appearance of fibroadenoma, or if the lesion is clearly palpable but not seen on ultrasound, the next step is mammography. The mammographic examination may be limited to imaging the ipsilateral breast if the lesion does not have suspicious sonographic features. For a solid lesion that has any malignant features on ultrasound, bilateral mammography is usually performed. For new solid palpable masses, needle biopsy is typically performed for diagnosis, even if the lesion has an appearance suggestive of a fibroadenoma.
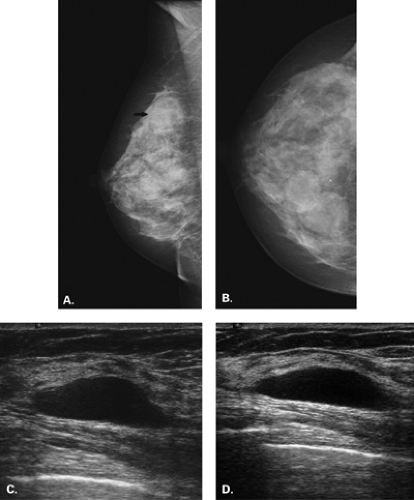
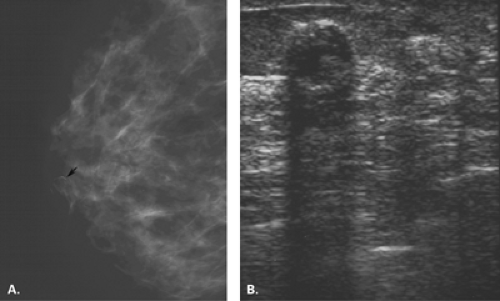
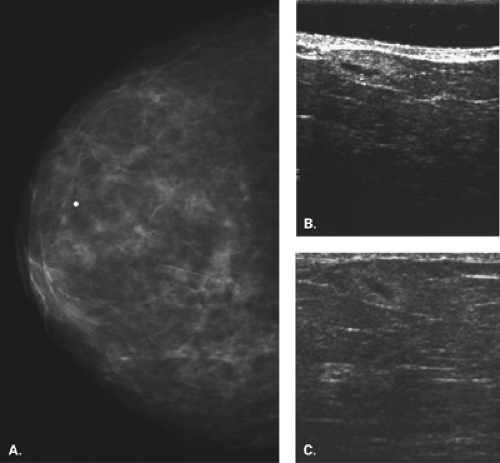
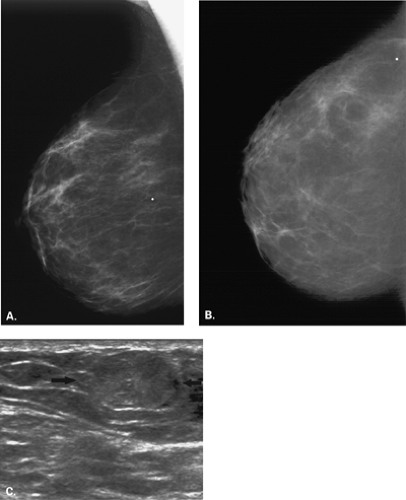
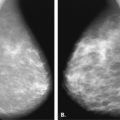
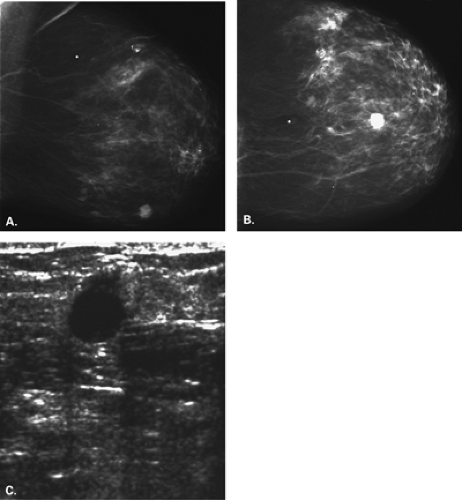 Figure 17.21 HISTORY: Screening mammogram on a 60-year-old woman. IMAGING: Right MLO (A) and CC (B) views show heterogeneously dense tissue and scattered benign calcifications. At 6 o’clock, there is a dense, round, relatively circumscribed mass. Ultrasound (C
Get Clinical Tree app for offline access
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree


|