Analysis of Calcifications
Some form of calcification is identified on the majority of mammograms. Many calcifications are clearly benign and require no further workup, whereas others are highly suggestive of malignancy. Between these two groups are various patterns of microcalcifications that are indeterminate in etiology and that often require biopsy for diagnosis.
Calcifications in breast tumors were described in early work by Leborgne (1) in 1951. In 1962, Gershon-Cohen et al. (2) described the forms of microcalcifications that were associated with breast malignancies as irregular in size and shape and ranging from minute to 3 mm in diameter. Approximately 50% of breast cancers are associated with calcifications (3), and as many as 98% of intraductal carcinomas contain microcalcifications (4). In cancers manifesting solely as microcalcifications, as many as 69% are noninvasive (5).
Since the early descriptions of breast calcifications, mammography has advanced, and higher image quality is attainable, thereby enhancing the visibility and detectability of microcalcifications. Publication of the American College of Radiology Imaging Network (ACRIN) trial of full-field digital mammography (6) has shown statistical significance in improved detection of breast cancer in three groups of women: those who are younger than 50 years of age, those who are pre- and perimenopausal, and those with dense breasts. In part, this improvement in detection of cancers may be reflected in the greater detectability of microcalcifications associated with ductal carcinomas.
When a radiologist is interpreting a mammogram, the decision that must be made when calcifications are identified is whether they are clearly benign, probably benign, indeterminate with a suspicion for malignancy, or highly suspicious for breast cancer. Although this assessment can be made on screening views in many cases, diagnostic mammography is often needed for microcalcifications. In particular, magnification views are performed for the complete evaluation of microcalcifications. Once the evaluation of the imaging features of calcifications has been made, a management plan can be recommended, such as routine follow-up annually, early follow-up at 6-month intervals, or biopsy. For those calcifications that are classified as suspicious and for which biopsy is recommended, the positive predictive value of malignancy is about 25% (7).
In order to accurately analyze small calcifications identified on routine screening mammography, additional evaluation is necessary. Magnification mammography is performed to more clearly depict their morphology, and supplemental views, including mediolateral and tangential views, may be performed to determine the answers to the following questions:
Are the microcalcifications real or artifactual?
What is the morphology of the calcifications?
What is the distribution of the calcifications?
Where are the calcifications located?
Are the microcalcifications clearly benign, such being dermal or milk of calcium?
Once these questions are answered, a management plan can be better determined.
Artifacts and Pseudocalcifications
There are a variety of foreign substances that when present on the skin may have the appearance of microcalcifications on mammography. The technologist must take care to inquire about the presence of such substances, and she should ask the patient to wipe the breast and axilla with a moist cloth if these substances may be present. If the “calcifications” are located very superficially, if they seem to conform to a pattern (such as within skin folds), or if they have a stippled appearance, they may represent pseudocalcifications or artifacts. In this case, the views on which the calcifications are seen should be repeated after skin cleansing.
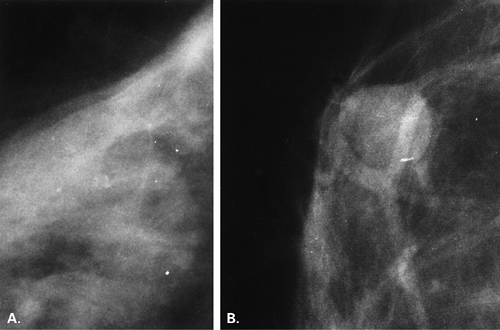
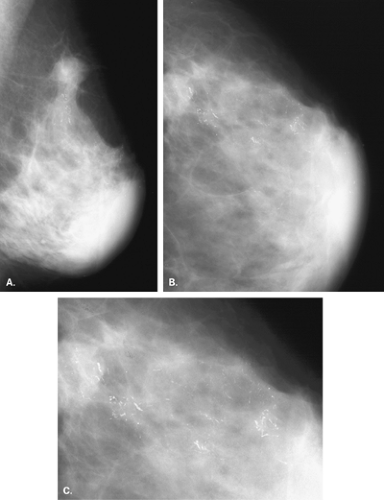
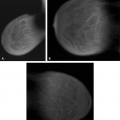
Substances on the skin (8,9) that can be evident as apparent microcalcifications on mammography include the following: deodorants (Fig. 6.1), bath powders, creams and lotions, ointments such as zinc oxide (Fig. 6.2), and
adhesive tape. Skin lesions, such as seborrheic keratoses, are particularly prone to trap any cosmetics that may be placed on the skin within the crenulations on their surface.
adhesive tape. Skin lesions, such as seborrheic keratoses, are particularly prone to trap any cosmetics that may be placed on the skin within the crenulations on their surface.
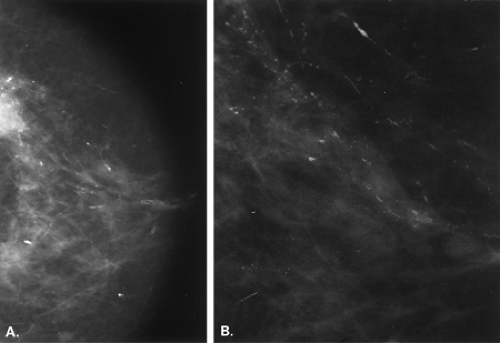
A cause of pseudocalcifications within the patient’s breast rather than on the skin surface is the deposition of gold in intramammary and axillary lymph nodes in patients with rheumatoid arthritis treated by chrysotherapy (10,11) (Figs. 6.3 and 6.4). Clinical history is key to confirming the diagnosis when this etiology is suspected. On mammography, the characteristic appearance is that of very fine, stippled “microcalcifications” within one or more intramammary or axillary lymph nodes. Additionally, the nodes may be mildly enlarged as a result of the rheumatoid arthritis.
Bullet fragments that are very small and scattered within the breast may have an appearance suggestive of calcifications, but because of the higher density of the metal, they appear more radiopaque than calcium. Other foreign bodies within the breast are usually not confused with true calcifications because of their defining shapes (i.e., surgical clips and markers, retained wire or hooks from needle localization procedures, sutures) and their overall very high density.
Other types of artifacts that may simulate microcalcifications on mammography are related to technical or nonpatient factors, including the following: mammographic equipment artifacts, cassette artifacts, and processing and film-handling artifacts. Most of these are unique to film screen mammography and are related to handling film. With full-field digital mammography, such artifacts do not exist. These artifacts usually have characteristic appearances that aid in differentiating them from true calcifications.
Scratches or “pick-off” from the processor occurs when the film emulsion is damaged as the film makes its transit through the processor, and such artifacts produce white specks on the film. Often these are brighter than true microcalcifications and therefore can be identified as artifactual. The processor must be properly maintained to minimize these.
Debris or dust between the film and screen within the cassette produces a white speck or pseudocalcification and is problematic not only by simulating calcium, but also by
producing focal blur from poor film screen contact. Cassettes and screens must be cleaned at least weekly according to standards established by the American College of Radiology’s (ACR) Mammography Accreditation Program (12), and in darkroom environments where dust control is a problem, more frequent cleaning is necessary to avoid these artifacts. Cassettes must be numbered so that when a film screen artifact is identified, the cassette may be pulled and recleaned.
producing focal blur from poor film screen contact. Cassettes and screens must be cleaned at least weekly according to standards established by the American College of Radiology’s (ACR) Mammography Accreditation Program (12), and in darkroom environments where dust control is a problem, more frequent cleaning is necessary to avoid these artifacts. Cassettes must be numbered so that when a film screen artifact is identified, the cassette may be pulled and recleaned.
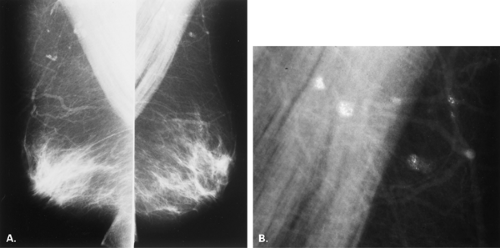
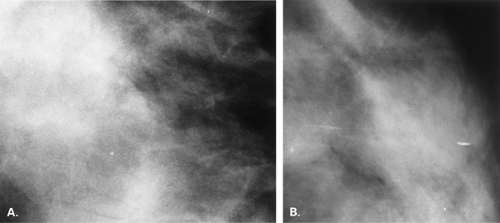
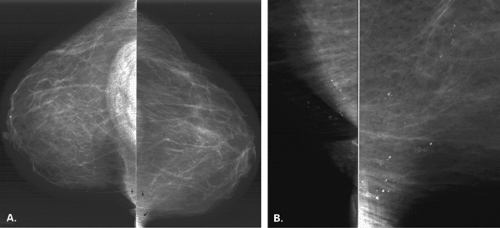
Other etiologies of white specks or pseudocalcifications relate to improper film handling. Fingerprints on the film occur when the technologist does not handle the film from the corner when loading the cassette. Often the pattern of the whorls in the fingerprint is evident, and the finding is seen on one view only (Figs. 6.5 and 6.6). One can repeat the view to confirm that the abnormality was artifactual without the unnecessary workup of using magnification views.
Mammographic Views to Evaluate Microcalcifications
Once it has been determined that calcifications are real, one must determine where they are located. A clock-face location (one o’clock, etc.) and a position or depth within the breast relative to the nipple (anterior, middle, posterior) is described. If the calcifications are seen on one view only, then additional views are selected to determine their position. If calcifications are seen on one view only and are not artifactual, they often are of dermal or milk-of-calcium origin. One should scrutinize the skin thoroughly to see if calcifications that seem to be within the breast on one view are actually on the skin on the other view.
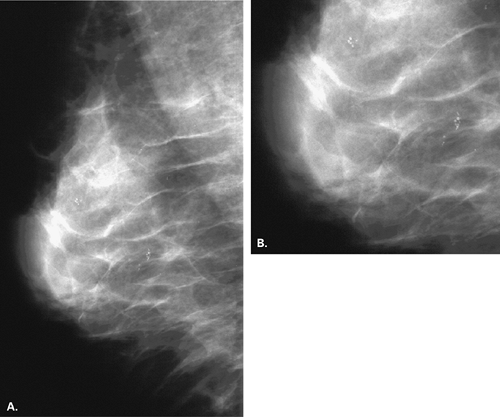
For calcifications that may be dermal, based on their morphology, but that do not project directly within the skin surface on one of the views, two methods of mammographic verification are available. The so-called bead-o-gram can be performed, in which the technologist, using a localization plate as is used in needle localizations, places a BB or radiopaque object over the calcifications on the view on which they are seen (13). The localizer plate is then removed, and a tangential view is performed with the BB in tangent to the beam. If the calcifications are dermal, they will project on the skin with the BB (Fig. 6.7). The second method is to perform stereotactic imaging (14). When dermal calcifications are targeted on a stereotactic pair of images, their z-position or depth will project at the skin surface. Caution should be used to not merely rely on the z-position of the
calcifications versus the compressed breast thickness to calculate that the calcifications are at the skin surface. Instead, a needle should be placed through the needle guides with the Δ Z at 0 mm to confirm that the needle tip is at the skin surface.
calcifications versus the compressed breast thickness to calculate that the calcifications are at the skin surface. Instead, a needle should be placed through the needle guides with the Δ Z at 0 mm to confirm that the needle tip is at the skin surface.
For parenchymal calcifications located very posteriorly on the mediolateral oblique (MLO) view and not seen on the craniocaudal (CC) view, exaggerated CC views may help to demonstrate their location on the transverse plane. For calcifications seen far medially and posteriorly on the CC view only, the lateromedial projection may demonstrate their position on the sagittal plane.
Once the location of calcifications is confirmed, a careful assessment of their morphology is undertaken. This is best accomplished with the use of magnification mammography (15). Because the assessment of the borders and shapes of individual calcifications is so critical to the determination of the likelihood of malignancy, the value of a high-quality magnification view cannot be overstated.
Magnification mammography requires the use of a microfocal spot (0.1 mm or less), to reduce geometric unsharpness; the breast is elevated on a magnification stand over the cassette, creating an air gap. Spot compression with collimation to the area of interest helps to reduce the scatter radiation and to displace the tissue, enhancing the image. Magnification in two projections, a CC and a mediolateral (ML), is most beneficial in the evaluation of microcalcifications because of the possibility of a diagnosis of milk of calcium.
For calcifications that are better seen, are more dense, or are more linear on the MLO than the CC view, a 90-degree lateral or ML magnification view is performed to determine if they layer and therefore represent milk of calcium in an area of cystic hyperplasia (Figs. 6.8 and 6.9). Because of subtlety of these differences on the MLO and CC views at times, magnification assessment of microcalcifications that are equivocal should include the ML projection.
If microcalcifications are assessed as “probably benign” with a likelihood of malignancy of less than 2% and are followed at more frequent intervals (i.e., at 6 months), the magnification views should be repeated at each mammogram in order to optimize comparison with the prior studies.
Analysis of Calcifications: Radiologic-Histologic Correlation
The Breast Imaging Reporting and Data System (BI-RADS®) of the American College of Radiology provides a lexicon (16) or nomenclature for the description of calcifications on mammography. The lexicon describes calcifications by morphology, distribution, and location and uses the following structure for overall patient assessment: Category 0: needs additional evaluation, i.e., magnification view; Category 1: normal mammogram; Category 2: benign finding, routine follow-up; Category 3: probably benign finding, early follow-up; Category 4: suspicious findings, recommend biopsy; and Category 5: highly suspicious for malignancy, recommend biopsy. Once the diagnostic evaluation of the patient is complete, calcifications identified on the mammogram are placed in one of the categories 2 to 5.
The assessment of calcifications identified on mammography should include the following points of evaluation: morphology, distribution, number, size, variability, interval stability in comparison with prior exams, and associated findings. Morphology of calcifications is the single most important feature, because it is related so closely to the pathologic or histologic finding. The anatomic location of many types of calcifications, particularly microcalcifications, affects their morphology, and thus their potential etiologies can be suggested.
Patterns of Microcalcifications: Benign Etiologies
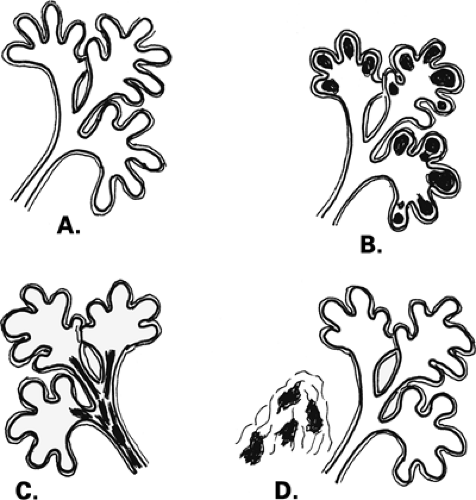
The terminal duct lobular unit (TDLU) is the basic histopathologic and physiologic unit (17) of the breast. The TDLU is the site of development of most benign and malignant conditions, including fibrocystic changes, fibroadenomas, and ductal and lobular carcinomas. It is also within the TDLU that microcalcifications are secreted or deposited (Fig. 6.10). The shape and variability of the calcifications relates very much to their location within the TDLU. When microcalcifications are located in the ductules, the blind-ending pouches of the lobule (lobular calcifications), they often appear rounded, punctate, or pearllike, and they are relatively similar or uniform in size and shape. They may be tightly grouped in a floretlike appearance or may be scattered throughout the breast. Because these are associated with fibrocystic conditions, the underlying parenchyma may be relatively dense on mammography.
Fibrocystic changes occur in the TDLU. Fibrocystic changes comprise pathologically a group of benign proliferative disorders of the breast, including fibrosis, cysts, adenosis, sclerosing adenosis, epithelial hyperplasia, papillomatosis, atypical hyperplasia, apocrine metaplasia, and chronic inflammation. Clinically, the patients are most commonly in the 30- to 50-year-old group and present with lumpy tender breasts that are cyclically symptomatic. Microcalcifications occur frequently in patients who have fibrocystic changes. The differentiation between ductal calcifications of fibrocystic origin and intraductal carcinoma is often not possible (18,19), and many of these clusters will need to be biopsied. About 15% to 30% of microcalcifications that are biopsied in asymptomatic patients are malignant (20,21,22), and the remainder are benign, mostly representing some form of fibrocystic change (23). Epithelial cells have the potential to secrete calcium actively, and indistinguishable deposits on mammography may be found in benign and malignant conditions. Lobular calcifications can be found in conditions that involve increased activity in the ductules, including adenosis, sclerosing adenosis, cystic hyperplasia, atypical lobular hyperplasia, and lobular carcinoma in situ (LCIS).
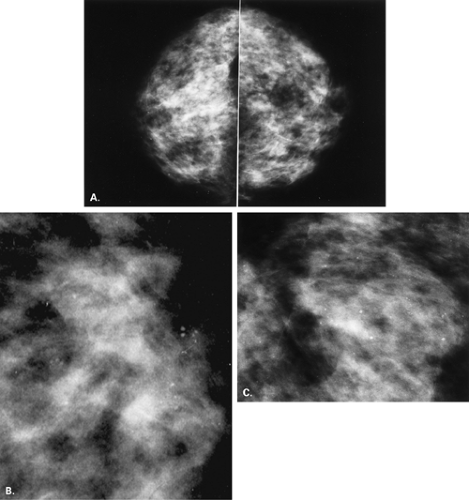
Sclerosing adenosis and adenosis often present as a diffuse process involving both breasts, with increased density and fine nodularity. In adenosis there is an increased
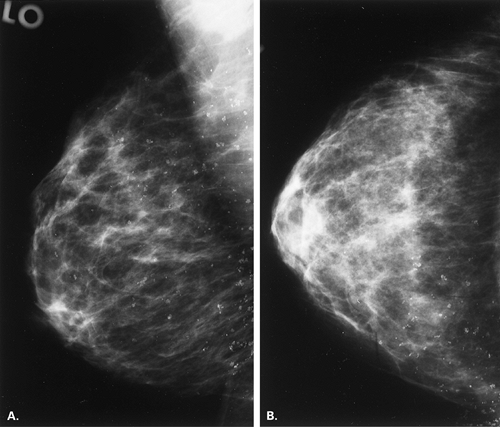
number of ductules within the lobule. The calcifications that occur in this condition are in the lobule, in the blind-ending ductules; therefore their shapes are punctuate, round, smooth, or granular (24). The process is often diffuse, and the calcifications may be in single or multiple groups, or they may be diffusely scattered and bilateral (Fig. 6.11). If the process is localized, however, the calcifications may be clustered, and biopsy often is performed to confirm the histology (24).
number of ductules within the lobule. The calcifications that occur in this condition are in the lobule, in the blind-ending ductules; therefore their shapes are punctuate, round, smooth, or granular (24). The process is often diffuse, and the calcifications may be in single or multiple groups, or they may be diffusely scattered and bilateral (Fig. 6.11). If the process is localized, however, the calcifications may be clustered, and biopsy often is performed to confirm the histology (24).
In sclerosing adenosis, there is lobular proliferation as well as fibrosis or sclerosis in the intralobular zone. The glands may grow haphazardly, producing an appearance
similar to that of tubular carcinoma (25,26). Because of the sclerosis, the ductules may be narrowed, and the calcifications may be quite small, appearing somewhat indistinct or amorphous. Like adenosis, sclerosing adenosis is often diffuse and bilateral, but this condition may be focal as well.
similar to that of tubular carcinoma (25,26). Because of the sclerosis, the ductules may be narrowed, and the calcifications may be quite small, appearing somewhat indistinct or amorphous. Like adenosis, sclerosing adenosis is often diffuse and bilateral, but this condition may be focal as well.
In the case of cystic hyperplasia, also a form of fibrocystic change, there is greater unfolding and enlargement of the ductules with the formation of microcysts. Calcium salts may be secreted into the fluid within the microcysts (27), and because the calcium is denser than the fluid, it lies in the dependent portion of the cyst. Therefore, when the patient is imaged in the CC projection, the calcifications lie in the bases of the microcysts and are visualized enface. Their appearance may be round or smudged, or they may not be seen. However, when the patient is imaged in the MLO projection, the calcifications are more dense or more apparent, and they may assume a linear shape. The confirmatory view is the ML magnification view, which is a true cross-table lateral projection. In this view, the calcifications layer in the dependent portion of the cysts and assume linear, crescentic, or the teacup shapes (27). The process may be extensive and bilateral, or it may be focal (28). Occasionally, larger macrocysts may also be associated with milk of calcium and show a dense layer of calcification on the ML view (29). Eventually, there may be complete calcification within the wall of the cysts, creating the appearance of small ringlike calcifications.
Papillomatosis, also known as duct hyperplasia of the common type, produces fine round or punctuate microcalcifications that extend over a large area of the breast (30). A dense prominent ductal pattern may be present and is associated with the fine microcalcifications. Occasionally, papillomatosis is focal or segmental, and biopsy is performed because of the distribution of the calcifications.
In periductal fibrosis, the calcifications occur in the stroma and may be diffuse or grouped. On histology, broad islands of fibrous tissue replace the normal fat of the breast. The calcifications of fibrosis may mimic intraductal calcifications found in ductal carcinoma in situ (DCIS) because they may be clustered, with irregular borders, and variable in size, shape, and density. However, the calcifications of fibrosis tend to be more coarse than is usually found in malignancy. The pattern of periductal fibrosis is that of dystrophic calcifications that develop in the stroma rather than in the ductal lumen.
Columnar cell changes have been more recently described as part of the spectrum of lesions involving the TDLU. The columnar epithelium of the ducts is altered with prominent apical cytoplasm snouts, intraluminal secretions, and varying degrees of nuclear atypia and architectural complexity (31). In a review of 100 breast biopsies performed for microcalcifications, Fraser et al. (31) found that columnar alternation with prominent apical snouts and secretion (CAPSS) was identified in 42% of cases. These lesions ranged from a more benign appearance of the epithelium to atypical lesions bordering on DCIS. Calcifications occurred within the CAPSS in 74% of cases, and these microcalcifications were frequently psammomatous. DCIS occurred with equal frequency with and without CAPSS, and when present with CAPSS was usually of the low-grade micropapillary or cribriform types.
Intraductal microcalcifications occur in epithelial hyperplasia, a proliferative disorder with the ducts. The work of Gallagher and Martin (32,33) with whole-organ specimens in patients with breast cancer showed that there is a spectrum of disease, from epithelial hyperplasia to atypical hyperplasia to carcinoma in situ, which occurs as nonobligate phases in the development of breast cancer.
Depending on the extent of the proliferative fibrocystic process, microcalcifications may be focal or extensive and may be present bilaterally. The calcifications are small (less than 1 mm in diameter) and may be irregular in form and variable in size and density, punctuate, or amorphous in appearance when associated with atypical lobular hyperplasia (ALH) or atypical ductal hyperplasia (ADH). Egan et al. (18) found the calcifications in epithelial hyperplasia to be indistinguishable from those of intraductal carcinoma. Stomper et al. (34) found that a majority of atypical hyperplasias were with or adjacent to another mammographic finding that prompted biopsy—often adenosis. A significant relationship existed between mammographic microcalcifications and ADH in these biopsy specimens that were otherwise benign.
The presence of hyperplastic lesions of the breast places a patient at higher risk for the development of carcinoma (35,36,37). In particular, atypical lobular hyperplasia increases the risk of developing breast cancer by approximately 6 times (35), and atypical ductal hyperplasia is associated with 4 to 5 times subsequent risk (36). Additionally, the combined factors of a family history of breast cancer and an atypical lesion on biopsy increase the risk of subsequent breast cancer by 11 times that of women with nonproliferative lesions and no family history (37).
Lobular Neoplasia
Lobular carcinoma in situ, or lobular neoplasia, was described by Foote and Stewart (38) as originating in the TDLU. This lesion is associated with other types of cancers and has a high propensity toward multicentricity and bilaterality (39). In a follow-up of 99 patients with LCIS treated by lumpectomy only, Rosen et al. (40) found that there was an equal risk of the patient developing invasive carcinoma in both the ipsilateral and the contralateral breast. Because about one third of patients with LCIS treated with excision alone will develop invasive carcinoma in either breast, the choices for therapy have ranged from lumpectomy only to bilateral mastectomies historically (40,41). Current management of most of these patients is excision only and mammographic surveillance.
The mammographic findings in LCIS are nonspecific and variable. However, grouped, round, lobular microcalcifications have been described in this condition (42). These calcifications are indistinguishable from those of lobular hyperplasia or focal sclerosing adenosis. Occasionally, in biopsies of lesions found on mammography in which the histology reveals LCIS, the calcifications may be located in the abnormal lobules (43), or they may be in adjacent benign lobules or areas of stroma (44,45).
Patterns of Microcalcifications: Malignant Etiologies
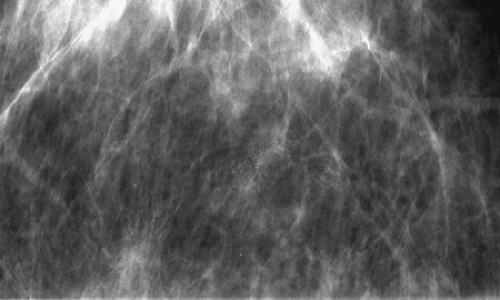
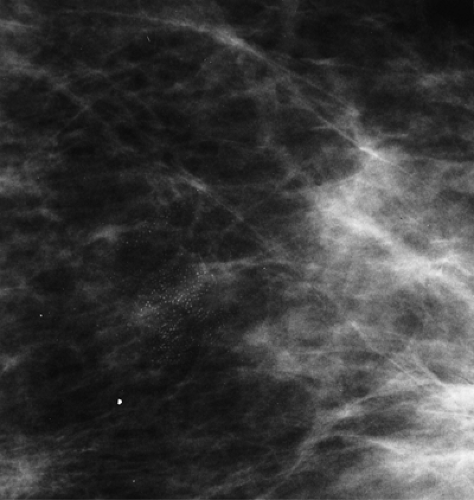
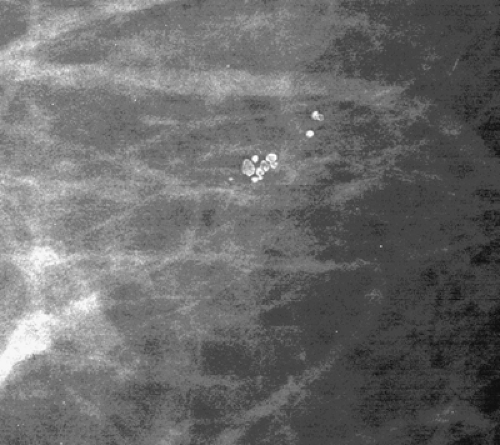
Microcalcifications that are of ductal origin are often found in the intralobular or extralobular (Fig. 6.12) terminal ducts, and their morphology and distribution reflect their location. Because the calcifications lie within the duct, which is a tubular structure, their distribution is often linear or segmental. Similarly, the individual microcalcifications may be linear or branching in form. Because the calcifications are deposited with the lumen of a duct that is lined by hyperplastic or malignant epithelium, their borders are often jagged or irregular, and there tends to be variability in their forms (pleomorphism), although small amorphous or granular calcifications may also occur in pathologic process in small ducts, specifically ductal hyperplasia, atypical ductal hyperplasia, and DCIS.
The formation of breast microcalcifications can occur by two processes: active cellular secretion of calcium salts or calcification of necrotic debris. Ahmed (46) described the concept of breast epithelial cells actively producing and secreting calcifications into the lumen of the ducts. Breast calcifications may be composed of calcium phosphate or calcium oxalate crystals. The calcium phosphate is readily visualized on hematoxylin and eosin (H&E)-stained slides as deeply purple. However, calcium oxalate crystals are not readily observed on H&E-stained slides (47). The use of polarized light microscopy is most helpful for the demonstration of the birefringent calcium oxalate crystals (47). Calcium oxalate, thought to arise as a secretory process in benign breast disease (48), produces colorless birefringent crystals (49). When no calcium is identified
on routine H&E staining, pathologists must consider calcium oxalate and use polarized light to evaluate the slides for this type of calcifications (49).
on routine H&E staining, pathologists must consider calcium oxalate and use polarized light to evaluate the slides for this type of calcifications (49).
Calcium phosphate crystals are basophilic and nonbirefringent, and they result from cellular degeneration in either benign or malignant disease. Radi (50) found that calcium oxalate accounted for 23% of mammographically detected microcalcifications on biopsy, and none were associated with carcinoma. Others, such as Fondos-Morera et al. (51), found that calcium oxalate may occur in breast cancers, but this type of calcification is more commonly found in benign lesions or associated with LCIS (50,52,53). Calcium oxalate has also been found (54) to be infrequently associated with DCIS.
Stomper et al. (55) found that in 100 cases of intraductal carcinoma, 72% presented as microcalcifications only, and 12% presented as nodules with calcifications. Other less common mammographic features of DCIS include circumscribed nodules, focal asymmetry, dilated ducts, ill-defined nodules, and focal architectural distortion (56). Because the calcifications of DCIS are deposited between crypts formed by irregular projections of epithelial cells, they form casts of the lumina of the ducts and, similarly, have irregular borders. Combinations of forms—including rod-shaped, punctuate, comma-shaped, tear-drop–shaped, Y or branching, and lacy calcifications—may be seen together and are considered of high suspicion for malignancy.
In a study of mammographic findings of various subtypes of DCIS, Evans et al. (57) found that calcification was present in 95% of patients with large-cell DCIS (often the comedo subtype) but in only 58% of patients with small-cell DCIS (solid, cribiform, micropapillary subtypes). Calcifications were found in 96% of cases of DCIS with comedonecrosis versus 61% of cases without necrosis. An abnormal mammogram without calcifications and mammograms showing predominately punctuate microcalcifications were seen more frequently in DCIS without necrosis (57).
The calcifications of comedocarcinoma are more likely to be linear, and this pattern is thought to be secondary to dystrophic calcification of intraluminal cellular debris and a high concentration of calcium in necrotic cells (57,58). Stomper et al. (55) found that linear microcalcifications were present in 47% of comedocarcinoma compared with 18% of the cribiform, solid, or papillary subtypes of DCIS. Granular microcalcifications were found in 53% of comedocarcinomas versus in 82% of the noncomedo subtypes. Barreau et al. (59) found a correlation between granular or linear branching calcifications and grade 3 histology with necrosis. These authors also found a correlation between a lesion being associated with greater than 20 calcifications and being grade 3 with comedonecrosis (59). Holland et al. (58) described the calcifications within well-differentiated DCIS to be small, laminated pearlylike particles in the luminal spaces within the tumorous ducts. Holland et al. (58) found that calcifications in poorly differentiated DCIS were linear, branching, or granular on mammography. The linear calcifications represented the three-dimensional image of the necrotic debris of calcium, which coalesced into casts of the center of the malignant ducts. Although in the majority of cases the calcifications associated with carcinoma are within the malignant ducts, Homer et al. (60) found that in one third of cases, the calcifications were both within the tumor and contiguous to it, and in 5% of cases, the calcifications were not associated with the tumor.
The size of individual calcifications is less important than their morphology for deciding their classification and potential etiology. The assessment of calcifications based on their size can be misleading, because malignancies are usually associated with microcalcifications of 200 μm or less, but this is certainly not always so. Comedocarcinoma, a subtype of DCIS, often manifests mammographically as casting, linear, branching microcalcifications, which can be larger than 200 μm and dense. A pitfall in the mammographic interpretation of microcalcifications is to dismiss somewhat large pleomorphic calcifications as being benign because of their size.
The most common distributions of malignant calcifications are in a focal cluster or group, or in a linear or segmental orientation. If the cancer is more extensive, the malignant calcifications may occur in a larger region of a breast or may even involve an entire breast. Egan et al. (18) found that carcinoma that presented as calcifications only was more often distributed as a cluster (66%); a mixture of scattered and clustered microcalcifications occurred in 34% of cancers. Microcalcifications that are diffusely scattered in all quadrants of both breasts are much more likely to be of benign origin. One should carefully scrutinize diffuse microcalcifications, searching for any cluster or area having a different morphology from the background pattern that might suggest a different etiology. One should also carefully review the mammogram for other clusters of suspicious calcifications when one group has been identified. DCIS has a great tendency to occur in multiple foci. Dershaw et al. (4) found that in 65% of breasts with DCIS, multifocality was present. In 41% of the cases, the maximum tumor expanse was greater than 2.5 cm, and all were multicentric. The detection of multifocal or multicentric carcinoma greatly affects surgical planning and patient management.
Zunzunegui et al. (61) found that in women with small, multifocal breast cancers with extensive casting calcifications and DCIS, the incidence of positive nodes was 33%, and there was a tendency for poor tumor markers. Thurfjell et al. (62) found a worse prognosis when small
invasive cancers presented as casting or pleomorphic calcifications. Stomper et al. (63) correlated mammographic features with pathologic findings of breast cancers manifested by calcifications only. In this study, 65% of patients had pure DCIS, 32% had DCIS with a focus of invasion, and 44% had invasive cancer. Invasion was more likely when calcification size was greater than 11 mm or morphology was linear, but invasion was not associated with an extent of calcifications greater than 10 mm.
invasive cancers presented as casting or pleomorphic calcifications. Stomper et al. (63) correlated mammographic features with pathologic findings of breast cancers manifested by calcifications only. In this study, 65% of patients had pure DCIS, 32% had DCIS with a focus of invasion, and 44% had invasive cancer. Invasion was more likely when calcification size was greater than 11 mm or morphology was linear, but invasion was not associated with an extent of calcifications greater than 10 mm.
Tabar et al. (64), in a study of invasive breast cancers less than 15 mm in size, found that the presence of casting calcifications was significantly associated with positive lymph node status, poorer histologic grade, and decreased survival. Twenty-year survival rates for women with 10 to 14 mm tumors were 52% when casting calcifications were present versus 86% to 100% for those women who had mammographic features that did not include casting calcifications.
Variability is size, shape, and density of microcalcifications is a worrisome feature (18), but variability must be assessed in conjunction with morphology. Those calcifications with sharp, jagged margins that are variable in appearance are much more likely to be malignant than are variably sized and shaped but smoothly marginated calcifications. DeLafontan et al. (65) found that vermicular shape, linear branching distribution, and irregularity of size of calcifications were strong predictors of malignancy.
Diffusely scattered, diffuse unilateral calcifications having spherical, hollow, or meniscoid shapes (usually associated with benign lesions) have been described (66) in ductal malignancies with apocrine features. However, in apocrine carcinoma, the pattern of microcalcifications is variable, composed of a mixture of milk of calcium with amorphous and pleomorphic microcalcifications that are unilateral. In cases of intraductal carcinoma in which there is retrograde involvement of the lobule (cancerization of the lobule), the lobular form of microcalcification may also be identified (67).
The greater the number of calcifications in an area, the more suspicious for malignancy (30,68,69). Egan et al. (18) found that 84% of breast cancers containing microcalcifications had more than 10 calcifications in a group. Millis et al. (3) found that in 33 cancers, 6 had less than five calcifications in a cluster. However, one should note that there is no definite distinction between less than versus more than five calcifications in a group and the need for biopsy. If microcalcifications are suspicious morphologically and less than five in number, biopsy should still be performed.
Interval changes from a prior mammogram with the development of new or increasing microcalcifications often warrant investigation with biopsy. However, morphology must be the primary consideration. New benign calcifications of fat necrosis, cystic hyperplasia, and dermal or vascular origin need not be evaluated further. On the other hand, stability of microcalcifications from prior studies of at least 2 years’ duration is a helpful indicator that the lesion is probably not malignant. Occasionally, DCIS may be manifested as calcifications that are stable for more than 2 years (70). Because of this, calcifications that are suspicious in morphology but stable on mammography may still need to be biopsied.
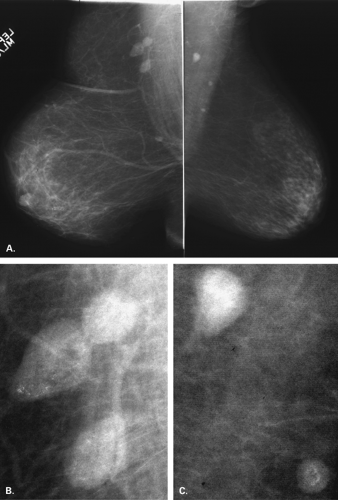
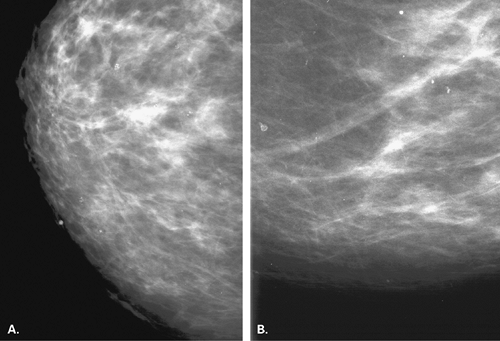
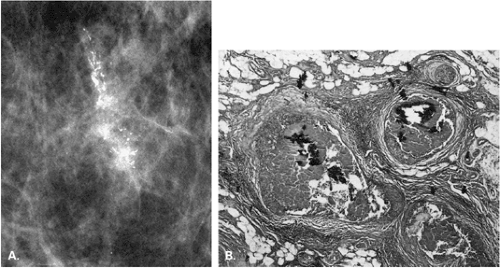
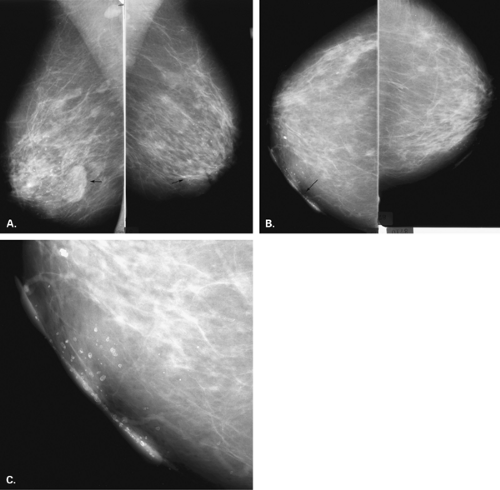
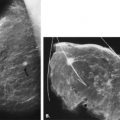
The identification of an extensive intraductal component (EIC) associated with an infiltrating cancer may be a predictor of a high risk of finding residual carcinoma on re-excision (71) and a higher local recurrence rate after breast conservation therapy (lumpectomy and breast irradiation) (72). An EIC is defined (73) as the presence of DCIS occupying an area of ≥25% of the area occupied by the infiltrating cancer and the presence of DCIS beyond the edge of the infiltrating tumor. Tumors detected only by mammography or tumors presenting as microcalcifications are more likely to be EIC positive, but this preoperative analysis is not absolute (Figs. 6.13 and 6.14) (74).
The presence of an extensive intraductal component is a major factor for predicting local recurrence after breast conservation and radiotherapy. The presence of malignant-appearing microcalcifications with or without a mass has been found to be associated with an extensive intraductal component, and when the calcifications extend over an area greater than 3 cm, the likelihood of EIC positivity is significantly increased (75).
Patterns of Calcifications as Defined by the BIRADS Lexicon
The ACR BI-RADS® lexicon (16,76) defines microcalcifications based on morphology and distribution. Those calcifications having a benign morphology include the following patterns: skin, vascular, coarse, dystrophic, large rodlike, eggshell or rimlike, lucent-centered or spherical, sutural, milk of calcium, round, and punctate.
Skin Calcifications
Skin calcifications are typically single or grouped, very-well-demarcated, small, round, spherical, or polygonal calcifications (77). These usually appear to be very superficially located because of their dermal origin, although they may superimpose over the parenchyma on the two standard views. They typically occur in sebaceous glands and are related to chronic inflammation (78). Because of their location within the interconnecting lumina of the sebaceous glands, their pattern may be in the form of a paw print. Another sign that calcifications may be dermal is the “tattoo sign” described by Homer et al. (79). In this case, the calcifications lie in a fixed orientation relative to each other on two different views, indicating a superficial location.
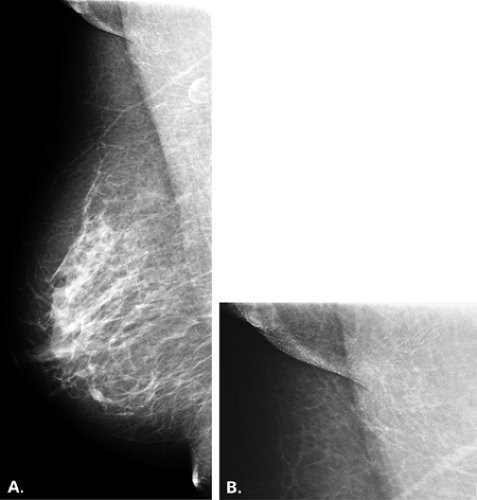
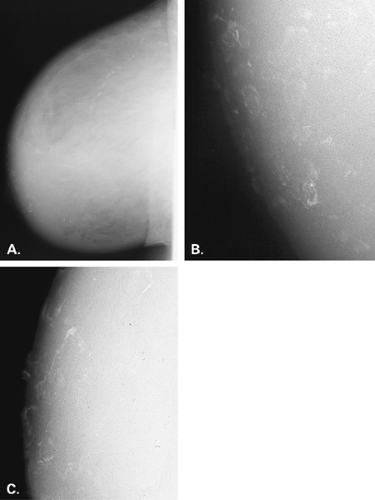
Dermal calcifications also may occur within or on the surface of skin lesions such as nevi, keratoses, and skin tags. Common locations for dermal calcifications are far medially and posteriorly in the cleavage area, within glands of the areola, and in the axillary region of breast (Figs. 6.15,Figs. 6.16,6.17). Calcifications of dystrophic origin may occur in skin that is scarred, such as at burn sites or within keloids. These calcifications are very well defined and round or spherical in form, and are often more dense than other skin calcifications (Figs. 6.18 and 6.19).
Vascular Calcifications
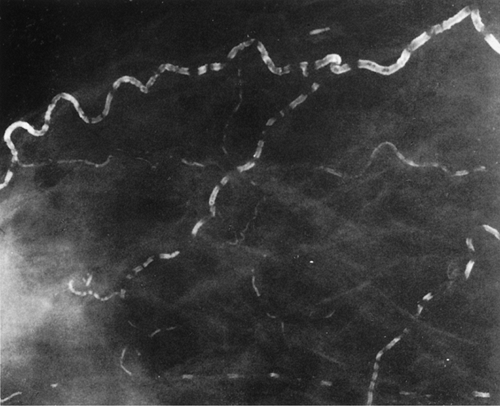
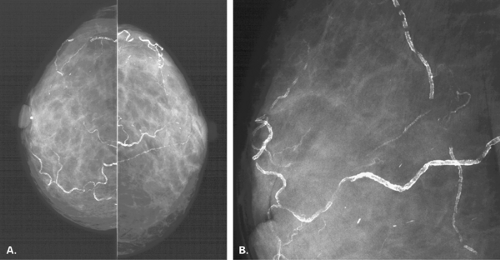
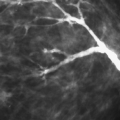
Vascular calcifications occur within arteries of the breast as they do elsewhere in the body. The most common etiologies of vascular calcifications are atherosclerosis and renal disease. Extensive calcification in the small vessels with the breast is usually associated with renal disease (80,81) and secondary hyperparathyroidism. A weak correlation exists between diabetes mellitus and the presence of vascular calcifications (82,83). Kemmeren et al. (84) found that breast arterial calcifications identified on mammography were associated with an increased risk of subsequent cardiovascular death in women older than 50 years and in diabetic women in particular. Moshyedi et al. (85) found that positive predictive value of breast arterial calcifications for coronary artery disease is 0.88, and the negative predictive value is 0.65, which is statistically significant. For women older than age 59, the correlation between vascular calcifications and coronary artery disease was not strong. Vascular calcifications are typically tramline or parallel lines of calcium corresponding to the calcified intima of the artery. These are usually circuitous and are not oriented in the pattern of the ducts toward the nipple-areolar complex (Figs. 6.20,Figs. 6.21,Figs. 6.22,Figs. 6.23,6.24). Early arterial calcifications may be stippled and fine, and the observation of the vessel corresponding to their path is key in suggesting the proper diagnosis. Magnification
mammography is often essential in this situation for differentiating early vascular calcifications from clustered fine microcalcifications of ductal or lobular origin (86).
mammography is often essential in this situation for differentiating early vascular calcifications from clustered fine microcalcifications of ductal or lobular origin (86).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree