The Augmented Breast
More than 250,000 women in the United States undergo an augmentation mammoplasty annually. Two studies (1,2) have reported the incidence of implants in American women to range from 3.3 to 8.1 per 1,000. The augmented breast presents a challenge to the radiologist to image the residual parenchyma adequately and to detect any abnormalities. The presence of implants may interfere with routine mammography and therefore has the potential to delay the diagnosis of breast cancer (3,4).
Fortunately, most women in the United States who have had augmentation mammoplasty have undergone placement of implants rather than direct silicone or paraffin injection. Augmentation may be performed for cosmetic reasons: to increase the size of both breasts unilaterally for an asymmetric hypoplastic breast, or after mastectomy for reconstruction. Augmentation procedures have included direct injection of silicone or paraffin into the breast, placement of a variety of types of implants in either a subpectoral or retroglandular location, and the use of several types of autologous myocutaneous flaps for reconstruction. In this chapter, the mammographic findings associated with the augmented breast will be discussed.
Direct Injection for Augmentation
Silicone injection for breast augmentation was never approved in the United Stated by the Food and Drug Administration (FDA) (5). Many patients seen in the United States with augmentation by direct injection have had the procedure performed in Asia or Mexico (6). Because of the intense response to the foreign material, management of the patient is quite difficult. Clinically, the breasts are quite hard or lumpy, and the exclusion of a tumor by palpation is impossible (6). Parsons and Thering (6) found that mastodynia was the most common presenting symptom in 28 patients with silicone-injected breasts. Another problem that these patients face is the tendency for the silicone to migrate far outside the breasts (7). Histologically, the silicone incites areas of fat necrosis, infiltration with fibrocytes, and cystlike spaces lined by fibrous tissues (7). There may be hyaline degeneration with calcifications of the inner surface of the fibrous capsule of the siliconoma (8). The calcification may represent deposition of calcium and phosphates near necrotic tissue (8).
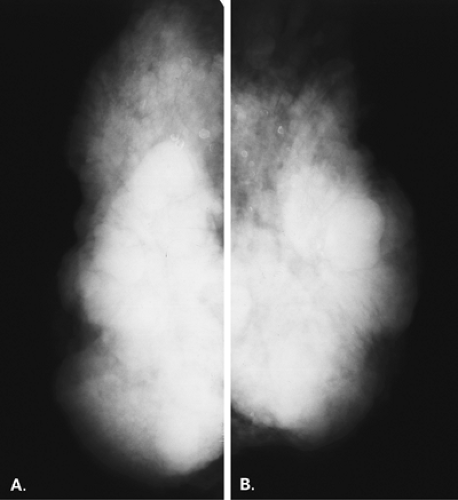
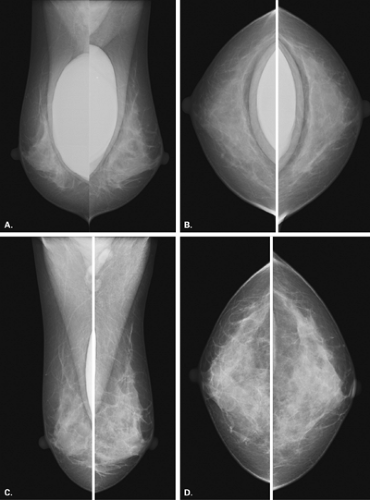
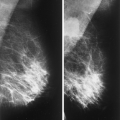
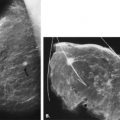
Mammography is markedly limited in patients who have undergone augmentation by injection, because the breasts are thick and hard and therefore difficult to compress well (Figs. 13.1 and 13.2). The injected substances and the fibrous response create a very dense appearance requiring a long exposure. Technically, by increasing the kVp to 30 or 32 or by using a tungsten target, one is sometimes able to penetrate the breasts adequately. Ultrasound shows numerous areas of dense shadowing related to the reaction to the injected substances. Because of this, sonography is usually not helpful to evaluate for malignancy.
Magnetic resonance imaging (MRI) can be very helpful to assess for tumors in a patient who has a compromised mammogram because of the density related to augmentation by injection. In a study of 16 patients who had undergone silicone injection breast augmentation and who underwent breast MRI, Cheung et al. (9) found that four of four cancers were accurately identified by using contrast enhancement techniques. In this series, the silicone granulomas were nonenhancing on MRI or were associated with a benign rimlike enhancement.
Koide and Katayama (8) found differences in the mammographic findings in breasts augmented by injection, depending on the substances used for injection. In patients who had paraffin injections, radiolucent masses were seen; 75% of these patients developed extensive small annular calcifications, and lymphadenopathy was common. In patients who received silicone injections, the mammographic nodules were of high density, and 29% of these women were found to have large, localized, eggshell calcifications in the breasts. Others (10,11) have found calcifications in patterns varying from irregular to small ringlike to eggshell shaped in patients with silicone injections.
Augmentation with Implants
Implants are most often placed for augmentation for cosmetic reasons, but also are frequently used for breast reconstruction after mastectomy. In patients with asymmetric breast size, a hypoplastic breast, or a chest wall deformity, an implant may be placed to achieve symmetry. Some of the types of implants used for augmentation have included saline filled, silicone gel, inflatable double lumen, and polyurethane coated (12,13). Commonly encountered silicone implants are those composed of a silicone gel contained within a silicone elastomer shell. The single lumen gel-filled implant can have a smooth or a textured shell; the textured shell was designed to reduce the incidence of capsular contraction (14). Another type of single-lumen implant that was designed to reduce capsular contraction is polyurethane covered. In this type of prosthesis, a thin layer of polyurethane foam is adherent to the gel-filled implant.
Saline implants are composed of a silicone elastomer shell that is filled with saline. These implants usually have a fill valve that is used for inflation of the implant. Double-lumen implants typically are composed of an inner lumen of silicone gel that is encased within an outer lumen of saline. Other double-lumen implants are reversed, with saline internally and silicone in the outer lumen.
When an implant is placed into the breast, the body reacts to the prosthesis by forming a fibrous capsule around it. This fibrous capsule surrounds the implant and can thicken and contract. The capsule can also calcify, which is visible on mammography and is often associated with capsular contraction (15). The use of implants with a textured surface or polyurethane coating has been found to decrease the severity of encapsulation (14).
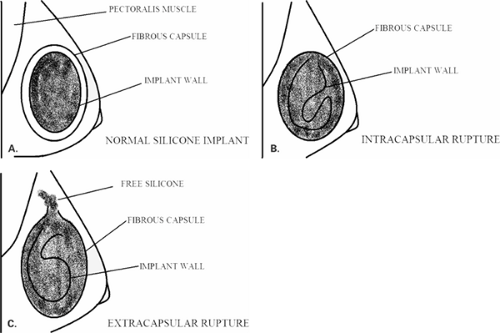
Goodman et al. (16) in a meta-analysis of literature evaluating implant rupture found that the estimated median life span of a silicone gel implant was 16.4 years. In the event of a rupture of a silicone implant, the intact fibrous capsule may contain the extruded silicone, so the contour of the implant both clinically and on mammography is unchanged. If the fibrous capsule also tears, the silicone can extend outside the capsule and into the surrounding breast tissue. This finding is termed an extracapsular rupture. The relationship of the implant wall to the capsule in the normal implant, in intracapsular rupture and extracapsular rupture is shown in Figure 13.3.
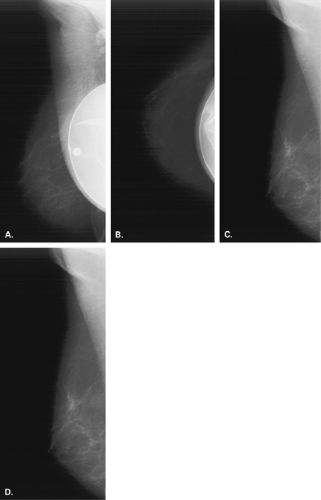
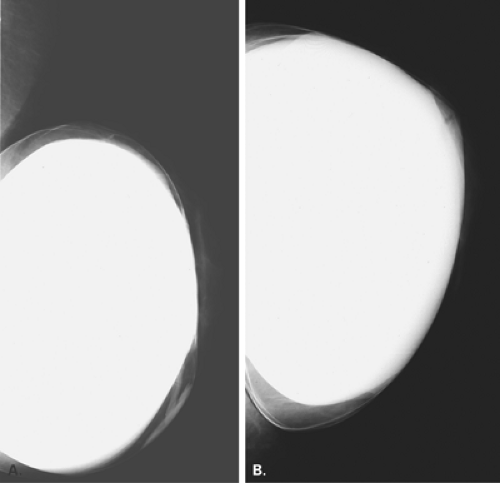
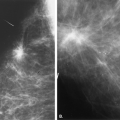
In the patient with a saline prosthesis, the implant is identified as saline filled because it is not of homogeneous density. The wall of the implant is more dense than the contents because the shell is a silicone elastomer that is more dense than the contained saline. Often folds and a fill valve are visible, and these are normal findings (Fig. 13.4). The density of a silicone implant is homogeneous because the wall and the contents are both silicone, and overall, the silicone implant is more dense than a saline implant (Figs. 13.5 and 13.6).
Positioning the Augmented Breast
Implants may be placed beneath the pectoralis major muscle or may be located anteriorly, in the retroglandular or prepectoral area. The imaging of a patient with implants is limited in that on routine mammography the prosthesis may obscure large areas of glandular tissue. The use of manual techniques (17) rather than phototiming is usually of help in imaging these patients.
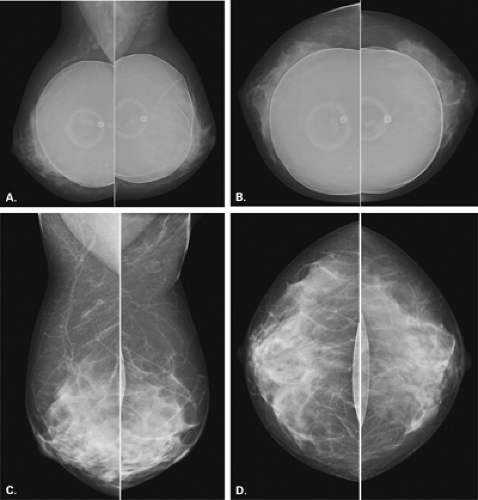
Eklund et al. (18) have described a modified positioning technique in which the implant is displaced posteriorly and the breast tissue is pulled anteriorly as compression is applied. This technique allows for improved compression and visualization of the parenchyma (Fig. 13.7). The implant-displacement views have become a standard part of the mammographic examination for patients with implants. This technique is more easily performed on patients with a moderate amount of native tissue over the implant or in patients with subpectoral implants. The description of Eklund et al. (18) for imaging the augmented breast includes (a) standard mediolateral oblique (MLO) and craniocaudal (CC) views using normal positioning techniques and (b) modified MLO and CC views with phototiming and implant displacement. In those patients who have encapsulated implants or in whom implant displacement is not successful, a third view, the lateromedial oblique (LMO), can prove useful in imaging the upper inner and the inner lower quadrants that are obscured on the routine views. Some authors also routinely perform a 90-degree lateral (mediolateral [ML]) view with the implant displaced (19). This may include tissue that may not be visualized otherwise on the MLO or CC implant-displaced view.
Implant Complications
Complications associated with implants include infection, hematoma formation, encapsulation, leakage or rupture, and collapse. In the immediate postoperative period following augmentation mammoplasty, infections or hematomas may occur. These are usually clinically evident, and ultrasound may be used for confirmation of a fluid collection. Treatment includes drainage and antibiotics if needed.
In the 2 to 3 weeks after an implant has been inserted, a fibrous capsule is deposited around it (12). This capsule may become fibrotic and contract, which is the most commonly associated complication with
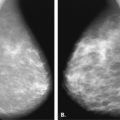
implants (20); encapsulation may occur in as many as 10% to 40% of patients (20,21). Retromuscular implants are much less likely to develop contractures (12). On mammography, the findings of a crenulated or irregular contour of the implant may suggest capsular contraction (13) (Fig. 13.8). A very rounded contour of the implant is also seen in patients with encapsulation. Irregularity of the implant contour may be palpated as a mass (11,13,22), but the irregular contour, as opposed to a parenchymal mass, can be differentiated with mammography and ultrasound if needed.
implants (20); encapsulation may occur in as many as 10% to 40% of patients (20,21). Retromuscular implants are much less likely to develop contractures (12). On mammography, the findings of a crenulated or irregular contour of the implant may suggest capsular contraction (13) (Fig. 13.8). A very rounded contour of the implant is also seen in patients with encapsulation. Irregularity of the implant contour may be palpated as a mass (11,13,22), but the irregular contour, as opposed to a parenchymal mass, can be differentiated with mammography and ultrasound if needed.
The capsule around the implant may become calcified. This is thought to be related to inflammation of the capsule and encapsulation. This is evident on mammography as curvilinear plaquelike calcifications (Figs. 13.9,13.10,13.11). Calcifications that are coarse and ringlike may also develop in the capsule of the implant and are indicative of an inflammatory reaction to the prosthesis (13) but not leakage of silicone. Irregularity of the contour of the implant may be caused by distortion or herniation of the prosthesis without a rupture (Figs. 13.12 and 13.13). Herniation has the appearance of a smooth bulge in the implant contour.
Rupture of an implant may be manifested clinically in a variety of ways. A saline implant that ruptures typically suddenly collapses, and there is an obvious decrease in the size of the breast. On mammography, the collapsed saline implant infolds on itself like a crumpled bag and the saline is absorbed (Figs. 13.14 and 13.15). The presentation is dramatic both clinically and on mammography. The rupture of a silicone implant may be associated clinically with pain, a change in contour of the implant, or a palpable mass.
The rupture of a silicone implant in the intracapsular space may cause a subtle change in contour of the implant but no obvious change in size or shape of the breast.
Mammography is typically normal, and the rupture is identified only on three-dimensional imaging, such as ultrasound or MRI. With an extracapsular rupture, the silicone extends beyond the edge of the capsule into the surrounding parenchyma, and this complication may be diagnosed on mammography as well as on ultrasound or MRI.
Mammography is typically normal, and the rupture is identified only on three-dimensional imaging, such as ultrasound or MRI. With an extracapsular rupture, the silicone extends beyond the edge of the capsule into the surrounding parenchyma, and this complication may be diagnosed on mammography as well as on ultrasound or MRI.
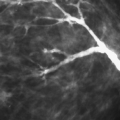
Palpable irregularity may also be associated with rupture of the implant with silicone extravasation. Mammography can identify the dense globules of silicone that may be calcified outside the contour of the implant, indicating rupture (12) (Figs. 13.16,13.17,13.18,13.19,13.20). The leaking silicone not only can form calcified nodules but also can present as intraductal casts of silicone (23) (Fig. 13.21). Silicone that is free in the breast is sometimes cleared by lymphatics and drains into the intramammary or axillary nodal chain. Nodes containing dense silicone may be seen on mammography and sometimes on ultrasound (Fig. 13.22). In patients with saline implants that were placed after a prior rupture of a silicone implant, residual free silicone that was not removed at surgery may be manifested clinically, mammographically, and on ultrasound (Figs. 13.23,13.24,13.25,13.26).
Role of Ultrasound
Sonography is most helpful for the evaluation of possible implant rupture, and ultrasound is best performed by using a linear array transducer. Scanning both superficially and deep is necessary to evaluate the entire implant and the
peri-implant space; this may require a lower power for the deeper area of the implant and a 5- to 7-MHz transducer (24). Scanning of the soft tissues and the axilla is also important to assess for signs of extracapsular rupture and free silicone.
peri-implant space; this may require a lower power for the deeper area of the implant and a 5- to 7-MHz transducer (24). Scanning of the soft tissues and the axilla is also important to assess for signs of extracapsular rupture and free silicone.
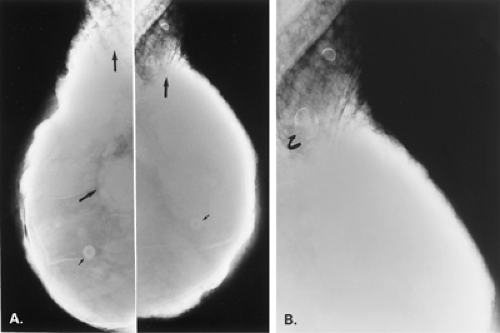
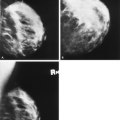
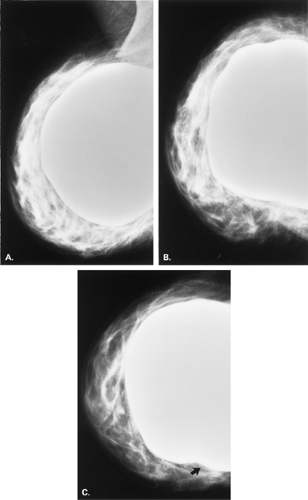 Figure 13.8 HISTORY: A 45-year-old gravida 2, para 2, abortus 1 woman after bilateral augmentation mammoplasty, with a smooth nodule in the left breast. MAMMOGRAPHY: Left MLO (A
Get Clinical Tree app for offline access
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree


|