IgGa
IgMa
IgG/IgM ratio
Mean
84.2
4.5
34.0
S.D.
82.8
7.1
43.6
Median
60.2
2.6
19.7
Range
0.2–588
0–135
0.2–482
The Composition of Circulating Modified LDL Immune Complexes and Diabetic Complications
Besides studying the pathogenic role of modified LDL antibodies [40, 49–51], we developed methodology that allows the measurement of modified forms of LDL and the corresponding antibodies involved in IC formation through the isolation and fractionation of circulating IC [36, 40, 41, 52]. This is an important methodological improvement over the direct assay of modified LDL or their corresponding antibodies in serum or plasma samples because most modified LDL in circulation is associated with the corresponding antibodies, and the measurements of either component of the circulating complexes is inaccurate due to the mutual saturation of antigen and antibody binding sites [36, 39, 52].
In contrast with the conflicting data generated by studies of modified LDL or antibodies to modified LDL [39, 53], data generated in clinical studies carried out on the DCCT/EDIC cohort (type 1 diabetes) with our assay have shown that high levels of oxLDL and AGE-LDL in isolated and fractionated IC are associated with increased risk for developing diabetic nephropathy [54]. Using coronary artery calcification (CAC) indices and carotid intima-media thickness (IMT) as endpoints indicative of cardiovascular disease progression we also found that increased levels of oxLDL and of AGE-LDL in circulating IC are associated in the DCCT/EDIC cohort with the development of coronary calcification and with increased levels and progression of carotid IMT. The levels of MDA-LDL in isolated IC show a significant but weaker correlation with increased carotid IMT [55, 56]. In contrast, in patients with type 2 diabetes (VADT cohort), the levels of oxLDL and AGE-LDL in circulating IC are not significantly associated with the occurrence of acute events, but high concentrations of MDA-LDL in IC are strong predictors of acute events, especially myocardial infarction (MI) [57]. In agreement with our data, Holvoet et al. reported in two separate studies a link between high levels of oxLDL and established CAD and between elevated plasma MDA-LDL levels and plaque instability [58, 59].
The correlation between MDA-LDL levels and plaque instability is particularly significant because it has been well-established that atherosclerotic plaque rupture is a critical event triggering thrombus formation, arterial luminal obstruction, and subsequent acute coronary syndromes [60]. Plaques that are prone to rupture consist of a larger intimal lesion with abundant macrophages and foam cells and a thinned fibrous cap [61]. Necropsy studies have demonstrated that atherosclerosis in diabetic patients is more extensive and accelerated than that in non-diabetic patients [62]. Furthermore, studies have also shown that atherosclerotic lesions in diabetic patients were more vulnerable as they had larger intimal lesions and more macrophage infiltration as compared to those in non-diabetic patients [63]. Analysis of gene expression in atherosclerotic plaques showed that when compared to stable plaques, vulnerable plaques have higher expression of matrix metalloproteinases (MMP) with collagenase activity, which contribute to the thinning of the fibrous cap, causing plaque instability and rupture [64]. Among the metalloproteinases, MMP-9 has been the object of considerable interest in recent years and according to some studies is an independent risk factor for atherothrombotic events [65, 66]. MMP-9 synthesis and release can be induced through TLR-4 stimulation, usually involving bacterial endotoxins [67] but also by minimally modified LDL [68]. The association of circulating MDA-LDL and IC-associated MDA-LDL specifically with plaque instability/acute CV events raises interesting questions such as whether IC containing different modified forms of LDL may lead to distinct gene regulation and in the case of MDA-LDL lead to plaque instability by inducing macrophage apoptosis and/or increased synthesis of matrix metalloproteinases, such as MMP-9 [69]. OxLDL-IC, in contrast, induce the release of proinflammatory cytokines [50] and promote collagen synthesis by smooth muscle cells [70], and therefore are more likely to contribute to atheroma progression without a significant effect on plaque stability (Fig. 10.1).
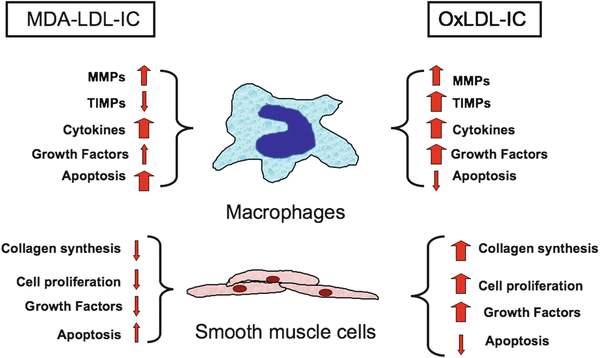

Fig. 10.1
Diagrammatic representation of the different effects of immune complexes prepared with human copper-oxidized malondialdehyde-modified LDL and the corresponding human antibodies reported by several groups (see text). While both types of immune complexes induce the release of pro-inflammatory cytokines, MDA-LDL-IC are pro-apoptotic while oxLDL-IC are anti-apoptotic and induce the release of proliferation and growth factors by macrophages and smooth muscle cells, and only oxLDL-IC induce collagen synthesis by smooth muscle cells
Considerable interest has been raised by the accumulation of apoptotic macrophages around the necrotic core of vulnerable plaques [69]. A variety of pro-apoptotic insults has been proposed to play a significant role in the evolution of atheromas, including oxidative stress, endoplasmic reticulum (ER) stress, accumulation of non-esterified (free) cholesterol, and effects of pro-inflammatory cytokines released by activated macrophages [69]. Accumulation of free cholesterol in macrophages in combination with signals delivered through scavenger receptors or with interferon-γ, known to be released by activated T lymphocytes in atheromas [15, 71], leads to serine phosphorylation of STAT-1 which is a critical element in the induction of apoptosis secondary to ER stress [72]. The apoptotic macrophages in atheromas are ingested by functional macrophages (efferocytosis). Efferocytosis in early lesions seems to result in suppression of inflammation, while in advanced lesions is associated with enhanced inflammation [69]. This evolution appears to be a result of defective efferocytosis, allowing the apoptotic cells to undergo necrosis, resulting in the accumulation of cell fragments that promote inflammation and plaque instability [69].
Pathogenic Mechanisms of Modified LDL IC
We have published extensive data proving that oxLDL-IC are more potent activators of human macrophages than oxLDL [50, 51, 73, 74]. The uptake of IC prepared with native or copper-oxidized LDL by human monocyte-derived macrophages is primarily mediated by Fcγ receptors, primarily FcγRI [75–77] and it has been shown that the binding of oxLDL antibody blocks the interaction of oxLDL with CD36 [78], so scavenger receptors are not involved in the process. The dependency of the vascular inflammatory process on the activation of phagocytic cells via Fcγ receptors has been demonstrated in double-knockout (DKO) mice generated by crossing apolipoprotein E-deficient mice (apoE(−/−)) with FcγR γ-chain-deficient mice (gamma(−/−)) [79]. The progression of atheroscleorosis in the DKO mice is significantly reduced in comparison with apoE(−/−) mice. For MDA-LDL IC and AGE-LDL-IC FcγRI is also involved but possible involvement of scavenger receptors or receptors for AGE-modified proteins has not been excluded.
One fundamental property of LDL-IC is their ability to deliver large concentrations of free and esterified cholesterol to macrophages [51, 75, 80]. The intracellular accumulation of free cholesterol is a known inducer of ER stress, which is believed to be the prime stimulus for the chain of events that results in modification of LDL and atheroma formation. However, experimental studies have shown that ER stress usually protects against apoptosis [69]. In fact, both oxLDL at concentrations not exceeding 75 μg/mL and oxLDL-IC prevent macrophage apoptosis [77, 81]. Whether the anti-apoptotic effect of oxLDL is a consequence of the induction of ER stress is not clear, because in addition to enhanced generation of reactive oxygen and nitrogen species [82], several other mechanisms seem to be involved, including the release of M-CSF mediated by the activation of a PI3K-dependent pathway, upregulation of the anti-apoptotic Bcl-XL gene by NFkB activation, activation of sphingosine kinase, which causes the levels of anti-apoptotic sphingosine-1-phosphate to increase, and inhibition of acid sphingomyelinase, which prevents pro-apoptotic ceramide generation [81, 83]. The anti-apoptotic effect is more pronounced with oxLDL-IC [77, 84] and is not unique to oxLDL-IC, because it has also been reproduced with KLH-anti-KLH IC [77]. However, there are significant differences between oxLDL-IC and other IgG-containing IC. Only oxLDL-IC can induce foam cell formation and the magnitude of the pro-inflammatory response induced in human macrophages is greater with oxLDL-IC than with KLH-IC, for example [50].
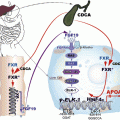
While oxLDL cell signaling is mediated by scavenger receptors, oxLDL-IC deliver activating signals via Fcγ receptors. The cross-linking of Fcγ receptors by IC induces phosphorylation of immunoreceptor tyrosine-based activation motifs (ITAMs) by kinases of the Src family, and consequent activation of the Syk pathway [85, 86]. Activation of Syk triggers the mitogen-activated protein kinase (MAPK) signaling cascade, which includes ERK1/2, p38 MAPK, and c-Jun N-terminal kinase (JNK). MAPK activation is also essential for Fc-mediated activation of NFκB [87]. Following the general rule, oxLDL-IC primarily engage FcγRI and induce the activation of the MAPK pathway [88], which is responsible for the expression of pro-inflammatory gene products. In addition, cross-linking of FcγRs by oxLDL-IC activates PI3K and c-Akt [77]. Activated c-Akt promotes cell survival by at least four different mechanisms: (1) phosphorylating the Bad component of the Bad/Bcl-XL complex which results in its dissociation and cell survival, (2) caspase 9 inactivation, (3) regulation of the expression of transcription factors, and (4) activating IKK kinases which phosphorylate IκB and, as a consequence, release the active form of NFkB, which induces the expression of genes favoring cell survival [89] (Fig. 10.2). The repertoire of oxLDL-IC-induced pro-survival genes is much wider than that induced by oxLDL alone [
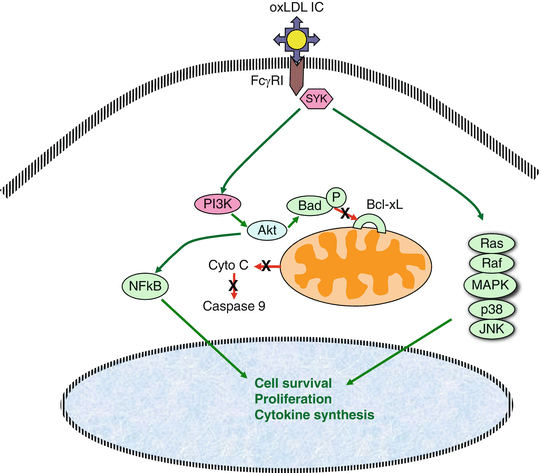
74]. Also, oxLDL-IC induce HSP70B expression in macrophages. This protein binds to the internalized lipid moiety of oxLDL-IC and prevents its degradation, while at the same time inducing sphingokinase-1 [82, 90].

Fig. 10.2
Diagrammatic representation of the activation pathways triggered by oxLDL-IC through the engagement of FcγRI. Two main pathways are activated, the MAPK pathway which is important for the activation of cell proliferation and cytokine synthesis, and the Akt pathway, which also contributes to the induction of cell proliferation and cytokine synthesis through NFκB activation and also promotes cell survival through the dissociation of the Bad/Bcl-XL complex, blocking the pathway that leads to the activation of caspase 9
In contrast to oxLDL, there is no published information concerning pathways of cell activation triggered by MDA-LDL or MDA-LDL-IC. The association of MDA-LDL with acute coronary syndromes [3, 59] and the association of high levels of MDA-LDL in the circulating IC isolated from patients with type 2 diabetes who had acute CVD events, mainly MI [57], strongly suggest that MDA-LDL and MDA-LDL-IC have proapoptotic activity. The different effects of cellular uptake of oxLDL-IC and MDA-LDL-IC (Fig. 10.1) could be a result of structural differences between MDA-LDL and oxLDL. The extent of MDA-lysine modification is much greater in laboratory produced MDA-LDL than in copper-oxidized LDL [52]. This difference results in the generation of epitopes unique to MDA-LDL, and the fact that MDA-LDL antibodies obtained by immunization of rabbits with laboratory-prepared MDA-LDL react with LDL isolated from IC proves that MDA-LDL with identical epitopes and, therefore, with similar structural characteristics, is generated in vivo. Also, while copper oxidation predominantly results in ApoB fragmentation, MDA modification is associated with ApoB aggregation [91]. Obviously, these differences in ApoB could determine different biological properties of the two forms of modified LDL. For example, it has been reported that the processing of heavily oxidized and aggregated LDL by macrophages is defective [92]. Thus, the uptake of MDA-LDL IC could result in a variety of conditions that could promote apoptosis, including: (1) the release of much higher concentrations of free cholesterol in the cell, (2) intracellular accumulation of aggregated LDL, (3) cytoplasmic release of lipoprotein degradation products and oxidized phosphatidylcholine, which could be transported to the extracellular compartment and then react with scavenger receptors and/or TLRs, delivering signals that would favor the activation of pro-apoptotic pathways.
There is considerable interest in identifying biomarkers indicative of plaque instability. A variety of proteins and enzymes have been proposed as candidates, as reviewed recently by Koenig. [93] Besides MMPs, reactive proteins (CRP), cytokines (IL-6, IL-18), enzymes (glutathione peroxidase, lipoprotein-associated phospholipase A-2 (Lp-PLA2)), myeloperoxidase, chemotactic proteins (monocyte chemotactic protein-1), and modified lipoproteins have been proposed as indicators of plaque instability [3, 58, 59, 66, 94, 95]. Our data suggest that modified forms of LDL can also be useful biomarkers for cardiovascular disease [54–56] and plaque vulnerability risk [57].
In conclusion, modified LDL plays a key role as a persistent insult leading to chronic vascular inflammation. The pro-inflammatory effects of modified LDL are significantly enhanced as a consequence of the formation of immune complexes as a consequence of the reactivity of different LDL modification with specific antibodies. In general, modified LDL IC have proinflammatory properties, but both clinical and experimental data suggest that there are differences in the consequences of cellular uptake of IC depending on the predominant type of LDL modification. This novel finding opens a variety of basic and clinical research perspectives, ranging from the investigation of the molecular mechanisms that are responsible for the different cellular effects of different LDL modifications to the definition of specific LDL modifications as risk factors able to discriminate between patients with different types or degrees of diabetes-associated complications.
References
1.
Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–70.PubMedCentralPubMedCrossRef
2.
3.
Holvoet P. Endothelial dysfunction, oxidation of low-density lipoprotein, and cardiovascular disease. Ther Apher. 1999;3:287–93.PubMed
4.
5.
Lopes-Virella MF, Virella G. Clinical significance of the humoral immune response to modified LDL. Clin Immunol. 2010;134:55–65.PubMedCentralPubMedCrossRef
6.
7.
Silverstein RL, Li W, Park YM, Rahaman SO. Mechanisms of cell signaling by the scavenger receptor CD36: implications in atherosclerosis and thrombosis. Trans Am Clin Climatol Assoc. 2010;121: 206–20.PubMedCentralPubMed
8.
Hoff HF, O’Neil J, Chisolm GM, Cole TB, Quehenberger O, Esterbauer H, Jurgens G. Modifica-tion of low density lipoprotein with 4-hydroxynonenal induces uptake by macrophages. Arteriosclerosis. 1989;9:538–49.
9.
10.
11.
12.
13.
Kugiyama K, Sakamoto T, Musumi I, Sugiyama S, Ohgushi M, Ogawa H, Horiguchi M, Yasue H. Transferrable lipids in oxidized LDL stimulate PAI-1 and inhibt tPA release from endothelial cells. Circulation Res. 1993;73:335–43.PubMedCrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree