TG
LDL-C
HDL-C
First author (ref. #)
Year published
N
Duration
Baseline TG
Placebo-corrected % change TG
Baseline LDL-C
Placebo-corrected % change LDL-C
Baseline HDL-C
Placebo-corrected % change HDL-C
Fenofibrate
Keech
2005
9,795
260
153.5
−23 %
118.5
−6 %
42.5
1 %
Vakkilainen
2003
405
172
225.6
−24 %
130.5
−9 %
39.6
3 %
Farniera
2005
253
12
276.5
−26 %
165
−3 %
42.5
16 %
Farniera
2005
372
12
274.3
−33 %
160.2
−5 %
42.5
15 %
Farniera
2007
244
12
231.1
−38 %
162.6
−11 %
45.3
19 %
Farniera
2007
367
12
226.4
−20 %
162.1
−1 %
44.7
1 %
Knopp
1987
227
24
193.1
−44 %
NR
NA
47.5
21 %
Davidson
2006
146
8
479.7
−37 %
119.3
16 %
35.7
16 %
Krempf
2000
138
13
127.9
−40 %
225.7
−33 %
56.8
4 %
Seidehamel
1989
147
8
614.6
−56 %
109.9
34 %
30.8
22 %
Nissen
2007
102
12
246
−37 %
NR
NA
37.4
15 %
Gemfibrozil
Frick
1987
4,081
262
176
−36 %
188.7
−9 %
47.3
9 %
Rubins
1999
2,531
52
160.5
−32 %
111.5
0 %
32
6 %
Frick
1993
628
260
183.2
−41 %
188.1
−11 %
46.3
10 %
Vinik
1993
442
20
272.3
−32 %
NR
NA
NR
NA
Schaeffer
1996
305
13
177.2
−42 %
206.5
−10 %
34.8
10 %
Avogaro
1999
217
20
317
−52 %
NR
NA
NR
NA
Wiklund
1993
137
12
159.8
−44 %
198.8
−14 %
46.3
19 %
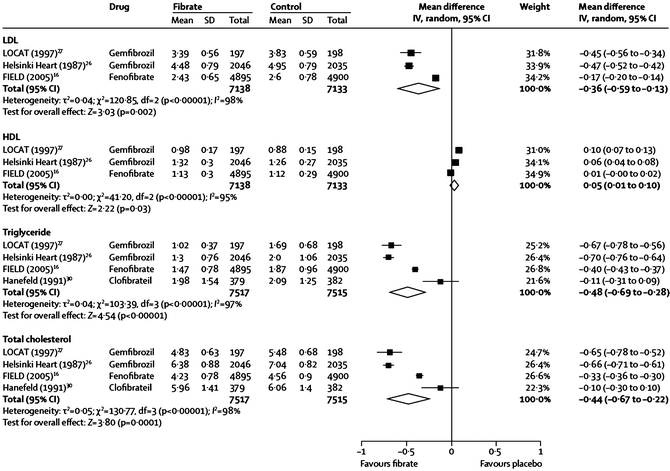
Fig. 20.1
Effects of gemfibrozil, fenofibrate, and clofibrate on major lipid parameters from a meta-analysis of several large randomized controlled fibrate trials. The name of the study or of the first author and the publication year are noted (the numbered references are from the publication by Jun et al. [46])
Fibrates may decrease, increase, or have a neutral effect on LDL-C levels, and baseline TG can be a very strong positive determinant of these changes (Fig. 20.2) [45]. The increase in LDL-C seen with fibrate use in the setting of a high baseline TG level likely relates to an increase in average LDL particle size (P < 0.001) [47–49], rather than due to an increase in LDL particle concentration. This is strongly suggested by the decrease in plasma apo B levels (P < 0.001), even when baseline TG is low [48], since there is one apo B molecule per VLDL, IDL, and LDL particle, and the vast majority of apo B-containing particles are LDL. Importantly, TG content of the large VLDL1 particles, which is a primary determinant of total plasma TG levels, is also directly related to the prevalence of SD LDL particles. This could be because large VLDL1 are direct precursors of SD LDL. More likely, however, it is because VLDL1 drives CE depletion and TG enrichment of the LDL core, followed by rapid lipolysis of that TG, resulting in a net shrinkage of the core, and of the entire LDL particle [44]. Thus, the ability of fibrates to reduce levels of plasma TG in general and VLDL1 in particular appears to correct two major aspects of the so-called atherogenic dyslipidemia common in DM-2: high TG levels and SD LDL [44].
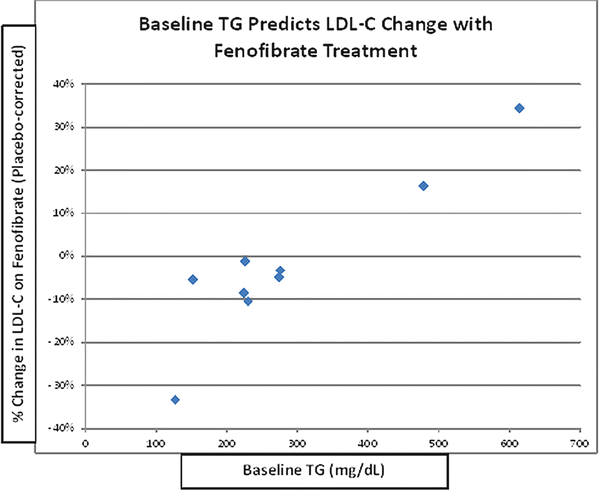

Fig. 20.2
Baseline TG predicts change in LDL-C with fenofibrate. Data from published randomized clinical trials analyzed by Abourbih et al. [45]
Low levels of HDL-C and of apo A-I clearly can result from HTG, by mechanisms similar to those for LDL size, noted above. That is, loss of CE and gain of TG by the HDL core is followed by rapid lipolysis of that TG, resulting in a net shrinkage of the core and of the entire HDL particle. Shrinkage of HDL results in exit of apo A-I from the particle, leading to rapid glomerular filtration and permanent catabolic loss of apo A-I from the plasma [38, 50]. Interestingly, however, the changes in HDL composition related to lower TG levels [38], and for that matter, niacin treatment [51, 52], which are larger particles, increased apo A-I and increased Lp A-I, are the opposite of those seen with fibrate use, as noted below. This suggests that fibrate-induced lowering of TG levels is not the mechanism by which fibrates raise HDL levels.
In addition to the strong impact of baseline TG levels on fibrate lipid effects, baseline HDL-C levels also may alter effects of fibrate treatment on major lipid parameters and HDL composition. Fenofibrate (160 mg/day) and simvastatin (40 mg/day) were given for 8 weeks in 52 patients, with moderate to very high CHD risk, selected for HDL-C levels <40 mg/dL [49]. Fenofibrate had dramatic effects on TG and HDL-C, with a 43 % decrease and 22 % increase, respectively, and baseline HDL-C was a strong inverse predictor of the HDL-C increase (R = −0.56, P = 0.003). Despite the large increase in HDL-C with fenofibrate, plasma levels of the major HDL protein, apo A-I, did not change. In the same study, plasma levels of Lp A-I particles (HDL with apo A-I but lacking apo A-II) actually decreased following fenofibrate treatment but were unchanged after simvastatin treatment. The HDL subclass distribution shifted towards smaller particles (significant increase in small HDL and decrease in large HDL) with fenofibrate treatment, but no changes in HDL size distribution were observed with simvastatin [49]. Other studies have confirmed these results that fibrate treatment causes a decrease in average HDL size and primarily an increase in apo A-II content [48, 49, 53].
Long-Term Effects of Fibrates on Lipids and Lipoproteins
An interesting paradox of fibrate therapy is a general lack of connection between lipid effects and CVD benefits. One manifestation is the lack of ability of lipid changes directly to predict reduction in CVD risk, as noted in the last section of this chapter. Another manifestation is that although lipid effects of fibrates may not be fully maintained throughout long-term clinical trials (see below), CVD effects tend to continue and even may increase with long-term treatment [54].
In the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) trial, in patients with diabetes, fenofibrate increased HDL-C levels modestly for the first 2–3 years after the start of treatment and then returned to near baseline levels by the end of the study [55]. There was a similar result of only partial long-term persistence of the initial HDL-C increase and TG reduction in the ACCORD-Lipid trial [56]. Interestingly, there appeared to be somewhat better preservation of HDL-C effects in the Helsinki Heart Study (HHS) with an average 11 % increase over the several years of the study [57]. Unfortunately, obvious possible explanations of the differences in persistence in long-term lipid effects among these studies, such as differences in patient compliance, do not appear to explain the discrepancies in degree and durability of lipid effects.
Lipid and Lipid-Related Effects of Fibrates vs. Statins or in Combination with Them
The lipid effects of fibrates tend to be complementary to those of statins, as has been noted in prior reviews of various studies [58, 59]. For example, lipid effects of fenofibrate (160 mg/day) were compared with those of simvastatin (40 mg/day), as noted above [49]. Fenofibrate had dramatic effects on TG and HDL-C, with a 43 % decrease and 22 % increase, respectively. In contrast, the TG and HDL-C changes were far less with simvastatin (−15 % and +6 %, respectively). Conversely, simvastatin significantly reduced LDL-C and total cholesterol levels (−28 % and −19 %, respectively) whereas fenofibrate did not significantly affect these parameters [49].
Since the majority of dyslipidemic patients are treated with statins, the question of the additivity of fibrate effects on lipids to those of a statin is of great clinical importance. As noted in several studies and reviews [58–60], the lipid effects of fibrates tend to be additive (as well as complementary, as noted above) to those of statins. A special case of fibrate-statin interaction is the FIELD study, in which half the patients were randomized to double-blind fenofibrate treatment while statins, excluded at baseline, were given to a moderate number of subjects in a non-blinded “drop-in” fashion by non-study physicians as desired on clinical grounds. To attempt to assess lipid effects of fenofibrate monotherapy in FIELD, Hiukka et al. examined lipid parameter changes with fenofibrate treatment among subjects followed at the Helsinki site who did not have drop-in statin treatment during the 5 years of the study [48]. Mean age of these subjects was 62 ± 5.7 years and duration of diabetes averaged 6 years. Differences were noted between fenofibrate and placebo groups for total cholesterol (−18.7 %; P < 0.001), TG (−25.8 %; P < 0.001), and LDL-C (−20.5 %; P < 0.001). No significant differences were observed between fenofibrate and placebo groups for HDL-C levels. Part of the explanation for the lack of HDL-C increase may lie in the fact that the mean baseline levels of HDL-C for both fenofibrate and placebo groups (42.9 mg/dL) were already above the NCEP ATP-III cutoff for low HDL-C (<40 mg/dL) [61] and the fact that HDL-C increases with any agent are generally inversely related to baseline HDL-C. It must be remembered that due to the nonrandom nature of the statin drop-in treatment in FIELD, these subjects are likely not representative of FIELD subjects in general, and so interpretation of lipid effects of fenofibrate in FIELD is unavoidably problematic.
Effects of Fibrates on Lipoprotein-Related Factors of Cholesterol Transport
A major function of HDL appears to be the promotion of cholesterol removal or efflux from extrahepatic cells and then delivery of that cholesterol to the liver, where it can be excreted from the body, a process called reverse cholesterol transport. The initial step in this process appears to be through interaction of HDL with specific cell membrane transport proteins such as the ATP-binding cassette transporter (ABCA1) [62]. In contrast, the scavenger receptor B1 (SR-B1) is believed to play an important role in cholesterol transport from HDL to the liver as a last step of reverse cholesterol transport [18]. Using plasma from patients treated with fenofibrate or simvastatin (as a source of HDL), cholesterol flux between cells and lipoproteins was determined in macrophages for ABCA1-mediated efflux and in hepatoma cells for SR-B1-mediated flux (measured as efflux but presumably also reflecting the ability of HDL to mediate influx). ABCA1-mediated cholesterol efflux to plasma and HDL was significantly increased with plasma from fenofibrate—but not simvastatin-treated patients (Fig. 20.3) [49]. Conversely, SR-BI-mediated cholesterol flux was significantly increased with plasma from simvastatin—but not fenofibrate-treated patients (Fig. 20.3) [49]. Thus, a combination fenofibrate and statin therapy may be better than either drug alone to enhance the full process of reverse cholesterol transport from the periphery to the liver.
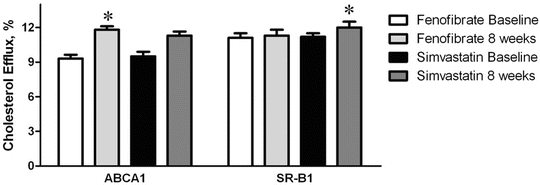

Fig. 20.3
Efflux of cholesterol through the ABCA1-mediated and SR-B1-mediated pathways to plasma from low HDL-C patients treated with fenofibrate or simvastatin. Asterisk denotes P = 0.015 for fenofibrate increase on ABCA1 from baseline and P = 0.016 for simvastatin increase on SR-B1 from baseline (figure is adapted from Franceschini, et al. [49])
Two additional factors related to HDL metabolism, concentration, composition, and particle size distribution and to reverse cholesterol transport are cholesteryl ester transfer protein (CETP) and lecithin cholesterol acyl transferase (LCAT). Fenofibrate and simvastatin are reported to significantly increase CETP by 17 % and 9 %, respectively [49]. In contrast, another study reported decreased CETP activity with fenofibrate and found that the decrease was related to increased LDL particle size and decreased coronary intimal hyperplasia after angioplasty and stent placement [63]. An explanation for the contrast between the two studies in the findings on fenofibrate effects on CETP activity is not readily available. LCAT may trend slightly, but nonsignificantly upward with fenofibrate and simvastatin therapy, by 7 % and 6 %, respectively [49].
Effects of Fibrates on Factors Related to Inflammation and Insulin Resistance
Increased inflammation is common in diabetes and appears to contribute to the excess CVD risk seen in this disorder. Several lines of evidence suggest that the inverse relationship between HDL-C levels and atherosclerosis may be mediated in part by an anti-inflammatory effect of HDL particles [64]. Thus, the low HDL-C levels often seen in DM-2 may be expected to contribute to the increased inflammation seen in this disorder. Further, the increase in HDL-C levels seen with fibrate therapy might be anticipated to have an anti-inflammatory effect in diabetes, as well as generally.
In addition to frequent low HDL-C levels, DM-2 is directly associated with increased levels of inflammatory biomarkers, including C-reactive protein (CRP) and lipoprotein-associated phospholipase A2 (Lp-PLA2) [65, 66]. Importantly, fibrate therapy can reduce levels of CRP and Lp-PLA2 [66, 67], as well as VCAM-1 and other inflammatory factors (see a recent review by Elkeles [68]), which presumably is a reflection of its anti-inflammatory effects. The effects of fibrate or statin monotherapy vs. their combination on inflammatory biomarkers in patients with DM-2 has been reported by Muhlestein et al., who studied 300 patients with diabetes and mixed dyslipidemia [60]. Treatment with fenofibrate, simvastatin, or combined therapy reduced hs-CRP by 14.1 % (P = 0.17), 16 % (P =0.04), or 15.9 % (P = 0.01), respectively (Fig. 20.4) and significantly decreased Lp-PLA2 by 26.9 %, 34.5 %, and 36.2 %, respectively (all P < 0.001, Fig. 20.4). Interestingly, combination therapy of fenofibrate with simvastatin had no additive effects on these markers despite the tendency towards additive effects (noted above) on lipid and lipoprotein parameters [60].
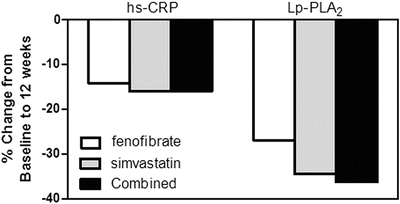

Fig. 20.4
Comparison of treatment with fenofibrate, simvastatin, and combined therapy on inflammatory biomarkers high-sensitivity C-reactive protein (hs-CRP) and lipoprotein phospholipase A2 (Lp-PLA2) after 12 weeks. Adapted from Muhlestein et al. [60]
Another effect of fibrates which is likely of clinical importance in treatment of patients with diabetes is their tendency to reduce insulin resistance, particularly in patients with high TG, low HDL-C, and other elements of the metabolic syndrome [69] (see also a recent review by Elkeles [68]), and it has even been suggested that fibrates be tested for a possible ability to prevent new-onset DM-2 [68]. Of equal or greater importance, the ability of fibrate therapy to reduce CVD risk may relate directly to the degree of baseline insulin resistance, as discussed below.
Effects of Fibrates on Microvascular Disease
Effects of fenofibrate on microvascular disease end points common in patients with diabetes have been explored in a meta-analysis [46] which included three recent trials of fenofibrate, the Diabetes Atherosclerosis Intervention Study (DAIS) [70], FIELD [55], and ACCORD-Lipid [56], as well as a small trial of etofibrate [71].
Beneficial effects of fenofibrate on certain aspects of diabetic retinopathy were reported in FIELD [72]. Although the pre-study primary end point of 2-step progression of retinopathy grade was not significantly reduced in the overall subject population (9.6 % with fenofibrate vs. 12.3 % with placebo; p = 0.19) or in those without pre-existing retinopathy (11.4 % vs. 11.7 %; p = 0.87), it was reduced substantially in patients with preexisting retinopathy (3.1 % vs. 14.6 %; p = 0.004) [72]. First laser treatment for retinopathy was required less often with fenofibrate than placebo (164 [3.4 %] vs. 238 [4.9 %] in placebo patients, respectively; hazard ratio [HR] 0.69, 95 % CI 0.56–0.84; p = 0.0002; absolute risk reduction 1.5 % [0.7–2.3]) [72]. Of likely importance, these effects were independent of traditional retinopathy risk factors of glycemia and blood pressure and, curiously, were also independent of on-study lipid levels. Also, a small trial of etofibrate (which has never been available in the USA), reported only in a German-language publication, showed reduced retinopathy [71]. A meta-analysis of the results of this trial and FIELD showed a highly statistically significant 47 % decrease in retinopathy (Fig. 20.5).
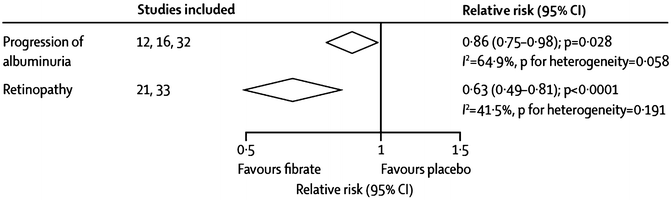

Fig. 20.5
Decreases in the microvascular end points of albuminuria and retinopathy with fibrate treatment. Adapted from a figure in a recent meta-analysis by Jun et al. [46]. Study references in the figure are as follows: “12” is ACCORD-Lipid [56], “16” is FIELD [55], “32” is DAIS [91], “21” is Emmerich [71], and “33” is a substudy of FIELD [72]
Regarding renal function, in a meta-analysis of three trials of fenofibrate, there was a statistically significant 14 % reduction in the risk of albuminuria progression (95 % CI 2–25 %; p = 0.028, see Fig. 20.5) [46]. Although the frequency of increased serum creatinine concentrations doubled (p < 0.0001), the absolute degree of increase was almost invariably modest in size and appears to be completely and rapidly reversible upon discontinuation of the medication, even after long-term use [46]. In FIELD, the largest of the three trials in this meta-analysis, there were 14 % fewer fenofibrate-treated subjects who had progression and 18 % more with regression of albuminuria vs. those on placebo (p < 0.001) [73]. Although plasma creatinine remained higher on fenofibrate than on placebo throughout the study, the chronic rate of rise was significantly slower (1.62 vs. 1.89 μmol/L annually, p = 0.01), with far less estimated age-related GFR loss (1.19 vs. 2.03 mL/min per 1.73 m2 annually, p < 0.001). Further, after fenofibrate washout at the end of the study, estimated GFR had fallen 72 % less from baseline on fenofibrate (1.9 mL min−1 1.73 m−2, p = 0.065) than on placebo (6.9 mL/min per 1.73 m2, p < 0.001), sparing 5.0 mL/min per 1.73 m2 (95 % CI 2.3–7.7, p < 0.001) [73]. Of particular interest, this greater preservation of estimated GFR with fenofibrate was seen primarily in subjects with either (1) baseline-high TG levels alone, (2) with baseline-high TG and low HDL-C together, or (3) TG reductions of ≥43 mg/dL on study drug. Curiously, however, progression to end-stage renal disease was not significantly reduced, occurring in 21 vs. 26 subjects with fenofibrate vs. placebo, respectively (p = 0.48) [73]. Thus, the overall net effect of fenofibrate on renal function in DM-2 appears to be at least modestly favorable. Of likely clinical importance and possible mechanistic meaning, these benefits are predicted by the same baseline lipid levels and on-treatment lipid changes as are the CVD effects (see below).
Lower extremity amputation is devastating complication of diabetes, which appears to be a result both of microvascular and macrovascular disease. In FIELD, any lower-extremity amputation was less often needed with fenofibrate than with placebo (45 vs. 70 events; hazard ratio HR 0.64, 95 % CI 0.44–0.94; p = 0.02, see Fig. 20.6) [74]. This finding was driven entirely by fewer “minor” (below the ankle) amputations (18 vs. 34 events; 0.53, 0.30–0.94; p = 0.027) with no difference between groups in “major” (ankle or above) amputations (24 vs. 26 events; 0.93, 0.53–1.62; p = 0.79, see Fig. 20.6) [74]. Interestingly, these effects of fenofibrate were seen primarily among patients without known large-vessel lower-extremity disease, and the benefits were unrelated to on-study lipid levels.
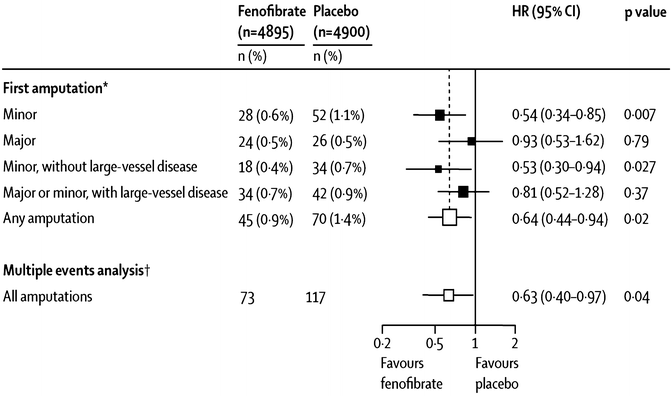

Fig. 20.6
Changes in frequency of lower-extremity amputations with fenofibrate in the FIELD study. For “first amputation,” patients are counted only once in each category. For the “multiple events analysis,” all amputations for each category are counted (Poisson method). “Minor” means amputations below the ankle; “major” means at or above the ankle. The figure is taken from Rajamani et al. [74]
Effects of Fibrates on Atherosclerosis
At least six studies have assessed effects of fibrate treatment on atherosclerosis. Three trials (two with fenofibrate and one with bezafibrate) have used carotid ultrasound for the measurement of carotid intima-media thickness (CIMT). In the St. Mary’s, Ealing, Northwick Park Diabetes Cardiovascular Disease Prevention (SENDCAP) Study, bezafibrate showed no effect on CIMT [75]. A similar lack of efficacy on carotid atherosclerosis was found with fenofibrate in the Helsinki cohort of the FIELD study [76]. A third study, however, found that over the 24-month study period, carotid wall thickness did not progress with fenofibrate, but did progress in the control group [77]. Of potential clinical relevance in explaining the differences among these studies, the two without evident carotid atherosclerosis benefit were exclusively in patients with DM-2, while the study showing a beneficial effect excluded DM-2 patients.
In contrast with the frequently negative findings in carotid atherosclerosis, particularly in patients with DM-2, fibrates consistently have been found to reduce atherosclerosis in the coronary arterial tree. Three published trials have studied the effects of fibrates on coronary atherosclerosis by quantitative angiography, with minimum lumen diameter (MLD) as the primary end point, and all three have reported favorable results. The first of these was a trial using bezafibrate [78] which found improvement in coronary lumen diameter, by quantitative coronary angiography, in young men after a myocardial infarction. The second study, the Lopid Coronary Angiography Trial (LOCAT) used gemfibrozil and found similar benefits [79]. The third trial, Diabetes Atherosclerosis Intervention Study (DAIS), used fenofibrate and also found improved coronary atherosclerosis [70]. It is of interest to note that this beneficial effect of fenofibrate on coronary atherosclerosis in DAIS was strongly related to its capacity to increase LDL particle size [47].
The mechanisms by which fibrates might tend to lack beneficial effects on carotid atherosclerosis, while in contrast, reproducibly reduce coronary atherosclerosis, are unknown. Interestingly, however, these regional differences in effects on atherosclerosis per se do correspond with a difference in regional effect on clinical events. That is, fibrates consistently reduce coronary heart disease events, but have little if any favorable effect on the cerebrovascular event of stroke, as discussed below. Curiously, in this regard, the one study showing reduced progression of carotid atherosclerosis is the only clinical trial of a fibrate to report reduction in stroke in an overall study population [77].
Fibrate Effects on Macrovascular CVD Events in General Study-Subject Populations
There has been no single randomized clinical trial of sufficient size and power to provide definitive data regarding effects of fibrate treatment on cardiovascular events. Consideration of individual trials can be instructive regarding certain specific aspects of this question, and some discussion of data from larger individual trials is included below, but the best assessment of the ability of fibrates to reduce CVD in general or any specific CVD end point comes from meta-analyses of available trials. Although trials can be difficult to pool due to differences in patient population, drug intervention, end points, etc., effective meta-analyses can be very instructive for the drawing of clinically relevant conclusions.
One large and fairly recent meta-analysis, by Abourbih and coworkers [45], looked at a total of 20 trials, using bezafibrate, fenofibrate, and gemfibrozil, with 25,655 subjects in nine and seven trials and 12,398 and 8,273 subjects using fenofibrate and gemfibrozil, respectively. Focusing on five trials with MI data, they found a significant 22 % decrease in nonfatal MI (Fig. 20.7a). In sharp contrast, focusing on six trials with mortality data, they found a nonsignificant trend towards a 5 % increase in all-cause mortality (Fig. 20.7b) [45].
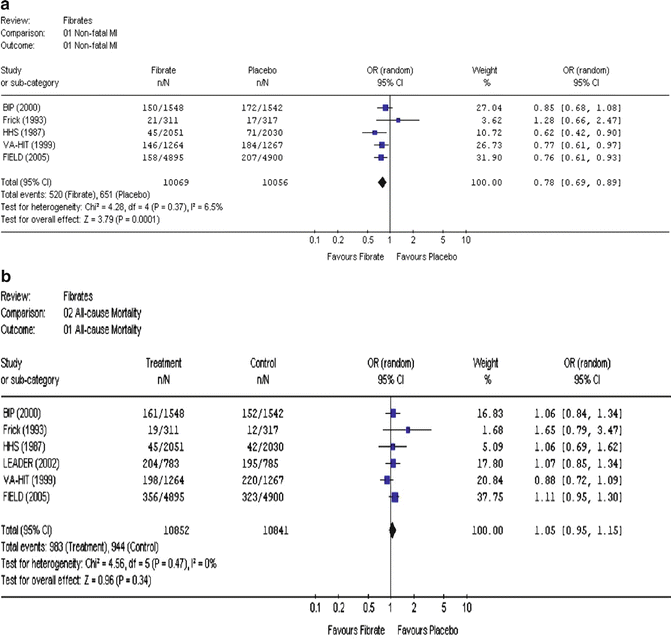

Fig. 20.7
Effects of fibrate treatment on the incidence of major clinical events: (a) nonfatal myocardial infarction (MI) and (b) all-cause mortality. The name of the study or first author and the publication year are noted. The figures are taken from the meta-analysis of Abourbih et al. [45]. OR odds ratio, CI confidence interval
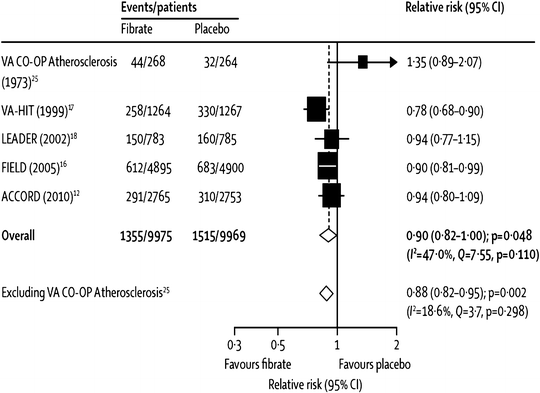
Another, more recent, meta-analysis, by Jun et al. [46], was the first (and only comprehensive one so far) to include the most recent and arguably most important trial of fibrate effects on CVD events, the ACCORD-Lipid study [56]. Due to differing trial selection methodology, some trials from the Abourbih meta-analysis were not included in the one by Jun, so both are discussed here. The Jun meta-analysis focused on studies selected for presenting CVD event data in at least 100 patient-years follow-up. These 18 trials included 45,058 subjects who had 2,870 major CVD events and 3,880 deaths [46]. Among five major trials with relevant data (two with fenofibrate and one each with clofibrate, gemfibrozil, and bezafibrate), there was a borderline significant (p = 0.048) 10 % decrease (relative risk, RR, of 0.90) in major cardiovascular outcomes, which became a highly significant 12 % decrease (p = 0.002) after exclusion of the one small clofibrate trial (see Fig. 20.8) [46]. Further, among 16 trials (six using clofibrate, three with gemfibrozil, four with bezafibrate, and three with fenofibrate), there was a highly significant 13 % decrease in coronary events, without evidence for heterogeneity among trials (p < 0.001, see Fig. 20.9) [46]. In further analyses of various cardiovascular events and other major end points, as noted in Fig. 20.10, pooled analysis of all studies with available data for each end point showed a highly significant 19 % decrease in nonfatal coronary events (p < 0.0001), but only nonsignificant trends towards reductions in sudden death and cardiovascular death (11 % and 7 % decreases and p = 0.2 and 0.1, respectively, Fig. 20.10). Similarly, there was a modest, borderline statistically significant trend towards a 10 % increase in nonvascular death (RR 1.10, p = 0.06), but there was no evidence for any benefit on total stroke (RR 1.03, Fig. 20.10) [46]. Curiously, with regard to stroke, one small clinical trial did report a statistically significant reduction in stroke with fenofibrate therapy [77]. Oddly, this trial does not appear in the Jun meta-analysis, an omission which may well have been inadvertent since it seems to have met the inclusion criteria [46].