INTRODUCTION
SUMMARY
The lymphoid tissues can be divided into primary and secondary lymphoid organs.* Primary lymphoid tissues are sites where lymphocytes develop from progenitor cells into functional and mature lymphocytes. The major primary lymphoid tissue is the marrow, the site where all lymphocyte progenitor cells reside and initially differentiate. This organ is discussed in Chap. 5. The other primary lymphoid tissue is the thymus, the site where progenitor cells derived from the marrow differentiate into mature thymus-derived (T) cells. Secondary lymphoid tissues are sites where lymphocytes undergo additional maturation and also interact with each other and with nonlymphoid cells to generate immune responses to antigens. These tissues include the spleen, lymph nodes, and mucosa-associated lymphoid tissues such as tonsils. The structure of these tissues provides insight into how the immune system discriminates between self-antigens and foreign antigens and develops the capacity to orchestrate a variety of specific and nonspecific defenses against invading pathogens.
Acronyms and Abbreviations:
AIRE, autoimmune regulatory gene; APECED, autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy; CT, computed tomography; GALT, gastrointestinal-associated lymphoid tissue; Ig, immunoglobulin; IL, interleukin; ILC, innate lymphoid cell; MALT, mucosa-associated lymphoid tissue; MHC, major histocompatibility complex; NK, natural killer; PALS, periarteriolar lymphoid sheath; PGA syndrome, polyglandular autoimmune syndrome; r, correlation coefficient; T, thymus-derived; TCR, T-cell receptor.
*This chapter was prepared by Thomas J. Kipps in the 8th edition and much of the text has been retained.
THE THYMUS
The thymus is the site for development of thymic-derived lymphocytes, or T cells. In this organ, developing T cells, called thymocytes, differentiate from lymphoid stem cells derived from the marrow into functional, mature T cells.1 It is here that T cells acquire their repertoire of specific antigen receptors to cope with the antigenic challenges received throughout one’s life span. Once they have completed their maturation, the T cells leave the thymus and circulate in the blood and through secondary lymphoid tissues.
The thymus is located in the superior mediastinum, overlying, in order, the left brachiocephalic (or innominate) vein, the innominate artery, the left common carotid artery, and the trachea. It overlaps the upper limit of the pericardial sac below and extends into the neck beneath the upper anterior ribs. It receives its blood supply from the internal thoracic arteries. Venous blood from the thymus drains into the brachiocephalic and internal thoracic veins, which communicate above with the inferior thyroid veins.
Arising from the third and fourth branchial pouches as an epithelial organ populated by lymphoid cells and endoderm-derived thymic epithelial cells, the thymus develops at about the eighth week of gestation.2 The thymus increases in size through fetal and postnatal life and remains ample into puberty,3 when it weighs approximately 40 g. Thereafter, the size progressively decreases with aging as a consequence of thymic involution.4 The cause of thymic involution is likely in part a result of the influence of glucocorticoid hormones.5 Nonetheless, there is evidence that T lymphocytes continue to develop throughout life, potentially including in some extrathymic sites.6
The volume of the thymus can be estimated by sonography. In one study of 149 healthy term infants within 1 week of birth, there was a significant correlation between the estimated thymic volume and the weight of the infant.3,7 However, no correlation was apparent between the estimated thymic volume and the infant’s sex, length, or gestational age. Also, there was no apparent correlation between estimated volume and the proportions of CD4+ T cells or CD8+ T cells found in the blood. The estimated thymic volume of healthy infants increases from birth to 4 and 8 months of age and then decreases.3 Most of the individual variation at 4 and 10 months of age appears to correlate with breastfeeding status, body size, and, to a lesser extent, illness. Breastfed infants at 4 months of age have significantly larger estimated thymic volumes than do age-matched formula-fed infants with similar thymic volumes at birth.8
A longitudinal fissure divides the thymus into two asymmetrical lobes, a larger right and a smaller left, that are derived from the right and left branchial pouches, respectively. These two developmentally separate parts of the thymus are easily separated from each other by blunt dissection.
Each lobe of the thymus is divided into multiple lobules by fibrous septa that extend inward from an outer capsule. Each lobule consists of an outer cortex and an inner medulla (Fig. 6–1). The cortex contains dense collections of thymocytes (developing immature T cells) that cytologically appear as lymphocytes of slightly variable size with scattered, rare mitoses. The lighter-staining medulla is loosely arranged and more sparsely populated by mature thymocytes and characteristic tightly packed whorls of squamous-appearing epithelial cells, called thymic or Hassall corpuscles (Fig. 6–2). These appear to be remnants of degenerating cells and are rich in high-molecular-weight cytokeratins. Hassall’s corpuscles are thought to serve a critical role in the development of regulatory T cells.9
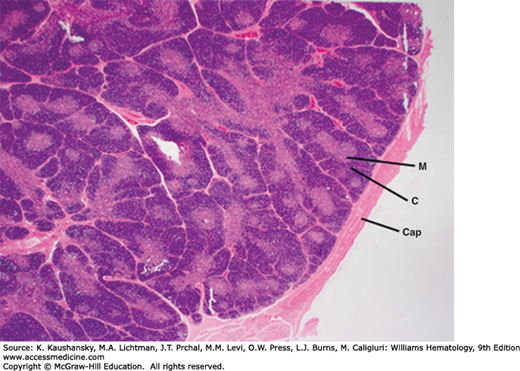
Figure 6–1.
Normal human infant thymus. The thymus is surrounded by dense connective tissue capsule (Cap). It is organized into adjacent lobules separated by capsular connective tissue extensions or trabeculae. The lobules each have a dense cortex (C) and a lighter staining medulla (M). The medulla is a continuous tissue surrounded by the cortex that extends throughout the thymus, and it cannot be appreciated in a single section. (Reproduced with permission from Lichtman’s Atlas of Hematology, www.accessmedicine.com.)
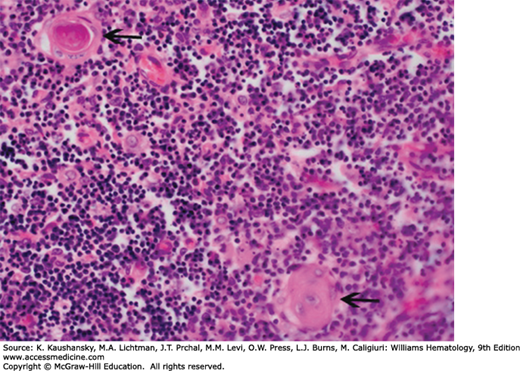
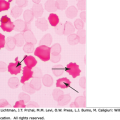
Figure 6–2.
Normal human infant thymus. Higher magnification. Medulla. The arrows indicate thymic corpuscles (synonymous with Hassall corpuscles). They are composed of tightly packed, concentrically arranged, type IV endothelioreticular cells with flattened nuclei. The central mass is composed of keratinized cells. In addition to thymic corpuscles and the mass of small densely stained T lymphocytes, the medulla contains scattered, larger, type V epithelioreticular cells with their light nuclei, dark nucleolus, and eosinophilic cytoplasm, evident on this section. (Reproduced with permission from Lichtman’s Atlas of Hematology, www.accessmedicine.com.)
The thymus contains several important cell types that serve a variety of functions including supporting the maturation of thymocytes into mature T cells. There are several types of specialized epithelial cells within the thymus.10 The three main categories of thymic epithelial cells are the medullary epithelial cells, which are organized into clusters; the cortical epithelial cells, which form an epithelial network; and the epithelial cells of the outer cortex. The epithelial cells in the cortex and medulla often have a stellate shape, display desmosomal intercellular connections, and likely function as support cells to developing thymocytes by providing important growth factors such as interleukin (IL)-7.11 In addition, at primarily the corticomedullary junction, the thymus contains marrow-derived antigen-presenting cells, mostly interdigitating dendritic cells and macrophages. Scattered B cells are also present in the thymus, and these interact with maturing thymocytes and potentially regulate T-cell development.12,13
After puberty, thymic involution begins within the cortex. This region may disappear completely with aging, while medullary remnants persist throughout life. Glucocorticoids also may induce atrophy of the cortex secondary to glucocorticoid-induced apoptosis of cortical thymocytes.5 This also may be seen in conditions that are associated with increases in circulating glucocorticoid hormones, for example, pregnancy or stress.14,15
The thymus is the site of T-cell development. The importance of the thymus is underscored by patients with DiGeorge syndrome, or chromosome 22q11.2 deletion syndrome, who lack the genes required for thymic development.16 These patients do not develop T cells and hence have profound immune deficiency.
Prothymocytes originate in the marrow and migrate to the thymus, where they mature into T cells (Chap. 76). Maturation of T cells is accompanied by the sequential acquisition of various T-cell markers including CD2, CD3, CD4 or CD8, CD5, and the T-cell receptor (TCR) (Fig. 6–3).17 Terminal deoxynucleotidyl transferase (TdT) is found in prothymocytes and immature thymocytes but is absent in mature T cells. TdT facilitates the successful rearrangement of TCR genes in immature thymocytes.18
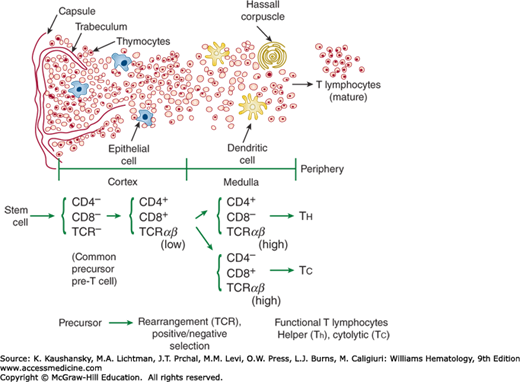
Figure 6–3.
Structure of the thymus. The top half of the figure provides a cross section of a thymic lobule, indicating the outer cortex (left), inner medulla (center), and periphery (far right). The arrows indicate various structures and cell types. As thymocytes mature, they migrate from the cortex toward the medullary region and acquire phenotypic features that are outlined at the bottom of the figure, as described in the text (Chap. 74).
T-cell precursors can be found in distinct microenvironments within the thymus. Marrow-derived CD34+ pre-T cells enter the cortex via small blood vessels and are double-negative for CD4 and CD8 antigens.1 One of the earliest identifiable T-cell membrane antigens is CD2. As the thymocytes proliferate and differentiate in the cortex, they acquire CD4 and CD8 antigens. They subsequently acquire the CD3 antigen and the TCR for antigen as they migrate toward the medulla. In the cortex, the thymocytes are induced to express the chemokine receptor, CCR7, which directs their migration to CCL19- and CCL21- producing cells in the thymic medulla where they undergo further maturation.19
Positive and negative selection of maturing T cells takes place in the thymus.20 Double-positive (CD4+ and CD8+) thymocytes undergo an initial positive selection step that is mediated exclusively by thymic cortical epithelium to ensure that developing T cells can recognize peptides in the context of self major histocompatibility complex (MHC) molecules.21 Thymocytes that have TCRs capable of interacting with self MHC molecules expressed by thymic cortical epithelial cells undergo expansion, whereas thymocytes with defective TCR undergo apoptosis.22,23,24 As these positively selected cells migrate toward the medulla, they experience negative selection through their interaction with thymic medullary epithelial cells in order to ensure that any T cells that react too strongly to self MHC molecules are deleted. These thymic medullary epithelial cells uniquely express the autoimmune regulatory gene (AIRE). AIRE encodes a transcriptional regulator that promotes ectopic expression of a large repertoire of transcripts encoding proteins that ordinarily are restricted to differentiated organs residing in the periphery.25 This allows the thymic medullary epithelial cells to express many different self-antigens, which are presented to developing thymocytes. Those thymocytes that have TCR that react too vigorously with the MHC molecules of the medullary epithelium will undergo apoptosis.23 Most of the developing thymocytes are destroyed. In this way, only those T cells that have the appropriate level of affinity for self-MHC molecules yet are not reactive against self antigens will reach the medulla to undergo the final maturation stages and eventually exit the thymus via efferent lymphatics as functionally competent naïve CD4+ and CD8+ single-positive T cells.
Patients with the rare disease autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) or polyglandular autoimmune (PGA) syndrome type I (PGA I) underscore the importance of negative selection of thymocytes by thymic medullary epithelial cells. APECED, or PGA I, is characterized by chronic mucocutaneous candidiasis, hypoparathyroidism, and adrenal insufficiency. In addition, most patients also have a number of other autoimmune manifestations, including thyroiditis, type 1 diabetes, ovarian failure, alopecia, and/or hepatitis.26 These patients have genetic defects in AIRE,27 which precludes their thymic epithelial cells from expressing the large variety of tissue differentiation self-antigens required for the negative selection of self-reactive thymocytes and the generation of central T-cell tolerance.25,28
THE SPLEEN
The spleen is a specialized abdominal organ serving multiple functions in erythrocyte clearance, innate and adaptive immunity, and the regulation of blood volume. In general the spleen contains two structurally and functionally distinct components: white and red pulp. The white pulp of the spleen consists of secondary lymphoid tissue that provides an environment in which the cells of the immune system can interact with one another to mount adaptive immune responses to bloodborne antigens. The splenic red pulp contains macrophages that are responsible for clearing the blood of unwanted foreign substances and senescent erythrocytes, even in the absence of specific immunity. Thus, it acts as a filter for the blood.
The spleen is located within the peritoneum in the left upper quadrant of the abdomen between the fundus of the stomach and the diaphragm. It receives its blood supply from the systemic circulation via the splenic artery, which branches off the celiac trunk, and the left gastroepiploic artery.29 The blood returning from the spleen drains into the portal circulation via the splenic vein. Therefore, the spleen can become congested with blood and increase in size when there is portal vein hypertension (Chap. 56).
Approximately 10 percent of individuals have one or more accessory spleens. Accessory spleens are usually 1 cm in diameter and resemble lymph nodes. However, they usually are covered with peritoneum, as is the spleen itself. Accessory spleens typically lie along the course of the splenic artery or its gastroepiploic branch, but they may be elsewhere.30 The commonest location is near the hilus of the spleen, but approximately 1 in 6 accessory spleens can be found embedded in the tail of the pancreas, where they may be occasionally mistaken for a pancreatic mass lesion.31
The average weight of the spleen in the adult human is 135 g (range: 100 to 250 g). However, when emptied of blood it weighs only approximately 80 g. On autopsy of 539 subjects with normal spleens, there was a positive correlation between the spleen weight and both the degree of acute splenic congestion and the subject’s height and weight, but not with the subject’s sex or age.32
The splenic volume can be estimated by computed tomography (CT) of the abdomen.33 In one study, the splenic volume was calculated from the linear and the maximal cross-sectional area measurements of the spleen, using the following formula: splenic volume = 30 cm3 + 0.58 (the product of the width, length, and thickness of the spleen measured in centimeters).34 Using this formula, the mean value of the calculated splenic volume for 47 normal subjects was 214.6 cm3, with a range of 107.2 to 314.5 cm3. The calculated splenic volume did not appear to vary significantly with the subject’s age, gender, height, weight, body mass index, or the diameter of the first lumbar vertebra, the latter being considered representative of body habitus on CT.
The splenic volume also can be estimated by sonography, which provides good correlation with volumes measured by helical abdominal CT or actual volume displaced by the excised organ. In one study of 50 patients, the linear measurement by sonography that correlated most closely with CT volume was the spleen width measured on a longitudinal section with the patient in the right lateral decubitus position (correlation coefficient [r] = 0.89, p <0.001). There was also good correlation between splenic length measured in the right lateral decubitus position and CT volume (r = 0.86, p <0.001). In another postmortem analysis of 32 normal adult spleens, the ultrasonogram measurements of maximal height, width, and breadth of the spleen were compared with the actual volume displaced by the excised organ.35 The mean actual splenic volume was approximately 148 cm3 (±81 cm3 SD), whereas mean splenic volume estimated from ultrasonography was 284 cm3 (±168 cm3 SD). Despite the differences between the actual and estimated volumes, these investigators did find a roughly linear correlation between actual splenic volume and the estimated splenic volume measured by ultrasound. However, there may be operator-to-operator variation in measurement of the estimated splenic volume, making the use of sonography in longitudinal studies technically demanding.
The spleen has an open circulation, which lacks endothelial continuity from artery to vein. When isolated spleens are perfused in washout studies, erythrocytes that appear in the splenic vein appear to be flushed out from three compartments. The red cells that are flushed out first come from a compartment that presumably is formed by the splenic vessels. The erythrocytes that are flushed out next come from a second compartment, where they presumably are loosely held within the filtration beds. The erythrocytes that are flushed out last presumably were adherent to cells of the filtration beds. Although 90 percent of the blood flow passes through the splenic vessels, only approximately 10 percent of the total splenic red cells are found within this first compartment. The second compartment is perfused by 9 percent of the total inflow yet contains 70 percent of the splenic red cells. The last compartment is perfused by only 1 percent of the inflow but contains 20 percent of the splenic red cells.
These compartments reflect the anatomy of the spleen and its stroma. The stroma is composed of branched, fibroblast-like cells called reticular cells. These cells produce slender collagen fibers, the reticular fibers, which are rich in type III collagen. The reticular cells and fibers form a meshwork, or reticulum, which filters the blood. Three major types of filtration beds can be distinguished by their structure and content: the white pulp, the marginal zone, and the red pulp.
The white pulp contains the lymphocytes and other mononuclear cells that surround the arterioles branching off the splenic artery. After the splenic artery pierces the splenic capsule at the hilum, it divides into progressively smaller branches. Each branch is called a central artery because it runs through the central longitudinal axis of a distinctive filtration bed that surrounds each central artery (Fig. 6–4). This is composed of a cuff of lymphocytes called the periarteriolar lymphoid sheath (PALS). The PALS is composed mostly of T lymphocytes, about two-thirds of which are CD4+ T cells. The PALS around white pulp arterioles of the human spleen is not continuous.36 Indeed, segments of the central arterioles might not be surrounded by T cells in areas where they run through lymphoid follicles containing pale areas of activated B lymphocytes interspersed with large, pale macrophages and dendritic cells.1 The migration of T cells to the PALS is governed by stromal cell production of chemokines, primarily CCL19 and CCL21, which interact with the chemokine receptor CCR7 that is expressed by naïve T cells.37 Stromal production of these chemokines can be stimulated by certain cytokines, such as lymphotoxin.38
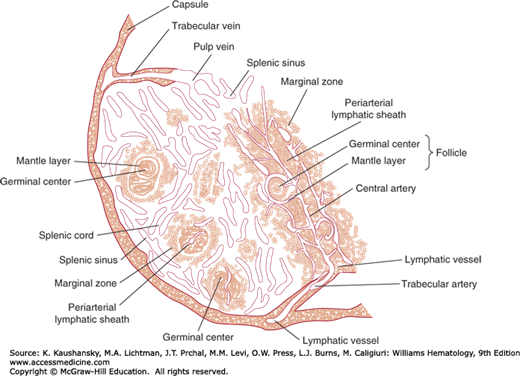
Figure 6–4.
Structure of the spleen. A branch of the splenic artery enters the pulp and becomes a central artery. Surrounding the central artery is a periarterial lymphoid sheath (PALS). At the circumference of the PALS is the marginal zone, which generally separates the white pulp of the PALS from the red pulp. Follicles of B cells with occasional germinal centers (malpighian corpuscles) are located at the outer margins of the PALS for the depicted central artery and the PALS of central arteries that are in a different plane from that of the figure.
On gross inspection of the surface of a freshly cut spleen, these follicles appear as white dots referred to as malpighian corpuscles (Fig. 6–5). These corpuscles contain a germinal center and have the same anatomic features and functions as secondary follicles in the lymph node. Branches coming off the central artery deliver disproportionate amounts of plasma and lymphocytes to the rim of the PALS (Fig. 6–6). These branches tend to run at acute angles, leading to a selective loss of plasma from the blood, a phenomenon referred to as “skimming.” After becoming relatively depleted of plasma, the arterioles then carry plasma-reduced blood into the filtration beds of the red pulp and marginal zone. As a result, the red pulp and marginal zone beds contain relatively high concentrations of red cells.