Fig. 17.1
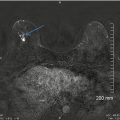
Long-term cosmetic outcome after breast-conserving surgery performed using a periareolar incision
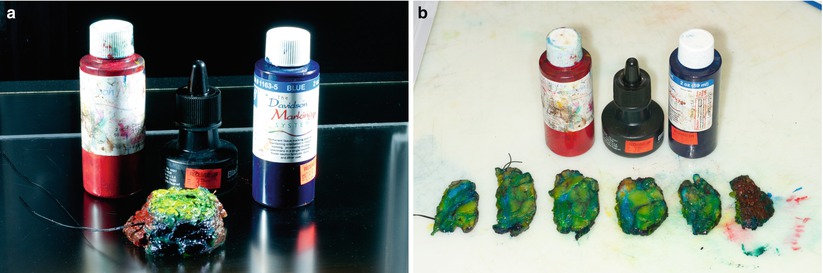
Fig. 17.2
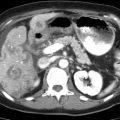
(a) Segmental mastectomy specimen shown after different colors of ink have been applied to designate the anatomic margins. (b) Segmental mastectomy specimen shown following inking and sectioning. Both the whole specimen and the sectioned specimen are radiographed, and careful examination is performed by the pathologist and the radiologist
Various techniques may be utilized to minimize contour defects following breast-conserving surgery. For larger defects, the deep parenchyma may be re-approximated. However, if a large cosmetic defect is anticipated preoperatively, it may be beneficial to involve a plastic surgeon to perform local tissue rearrangement and possibly a procedure on the contralateral breast to achieve symmetry.
The findings on the final pathology review dictate whether additional surgical therapy will be needed. At MD Anderson, margins are re-excised if the tumor is less than 2 mm from the inked margin. As discussed previously, inability to obtain negative margins after multiple re-excisions is an indication for mastectomy.
Mastectomy
Patients undergoing mastectomy for DCIS may be considered for total mastectomy, skin-sparing mastectomy with immediate reconstruction (Fig. 17.3a), or nipple-areolar-complex-sparing mastectomy with immediate reconstruction (Fig. 17.3b).
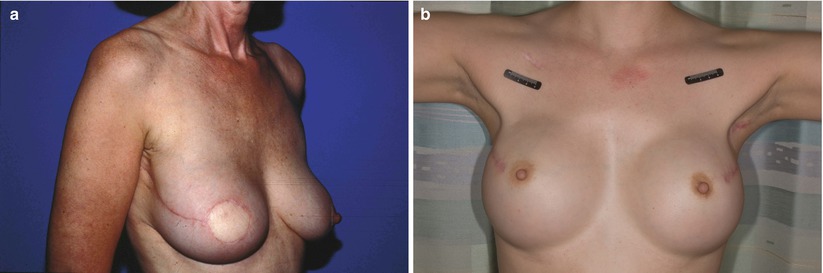

Fig. 17.3
(a) Skin-sparing mastectomy with TRAM flap reconstruction prior to nipple reconstruction. (b) Bilateral nipple-areolar-complex-sparing mastectomy with implant reconstruction
Although extensive DCIS is not a contraindication to skin-sparing mastectomy, patients with DCIS close to the skin may require excision of additional skin to achieve negative margins. Intraoperative specimen radiography is performed to determine the adequacy of margins. Excision of additional skin may be necessary if superficial disease is identified.
As discussed previously, careful selection of patients for nipple-areolar-complex-sparing mastectomy is crucial to optimize outcomes. A variety of incisions may be chosen for this type of mastectomy, including a radial incision, a lateral incision, or an inframammary incision. Incision placement may be dictated by the location of the tumor, prior biopsy scars, or patient or surgeon preference. Following excision of the breast tissue, the specimen is oriented, and clips are placed at the circumference of the areolar margin at the 3, 6, 9, and 12 o’clock positions as well as directly underneath the nipple to focus the pathologic examination. As with skin-sparing mastectomy, intraoperative specimen radiography is performed to determine the adequacy of margins. Excision of additional skin may be necessary if superficial disease is identified. If there is suspicion of disease in the tissue beneath the nipple, tissue from the area or areas of interest is subjected to intraoperative frozen section examination. The nipple-areolar complex should be excised if malignant cells are identified on frozen section examination.
Radiation Therapy
Radiation therapy is an important component of therapy for most women with DCIS who choose to undergo BCT. It is important to note that adequate surgical therapy is required to achieve superior outcomes with BCT. Radiation therapy cannot adequately compensate for inadequate surgery.
The benefit of radiation therapy for patients with DCIS undergoing breast-conserving surgery has been well established by prospective randomized trials. The NSABP B-17 trial included 814 patients with DCIS [13]. Following margin-negative tumor excision, patients were randomized to two groups, WBI and observation. Patients in the WBI group received 50 Gy to the whole breast without a boost to the tumor bed. Although there was no difference in OS between the WBI and observation groups at a mean follow-up time of 8 years, significant reductions were observed in the rates of both ipsilateral DCIS (12.1 % vs. 26.8 %, P = 0.007) and invasive recurrence (3.9 % vs. 13.4 %, P < 0.000005).
The EORTC 10853 trial included 1,010 patients with DCIS and was similar in design to the NSABP B-17 trial [14, 15]. Patients were randomized to WBI or observation after margin-negative tumor excision. As in the NSABP B-17 trial, patients in the WBI group received 50 Gy to the whole breast. However, in contrast to what was done in the NSABP B-17 trial, 5 % of patients in the WBI group received a boost to the tumor bed. At a median follow-up time of 10.5 years, no OS difference was seen between the two groups. However, patients randomized to postoperative WBI had fewer recurrences, including both DCIS and invasive recurrences, than patients randomized to observation (74 % vs. 85 %, P < 0.0001). It is important to note that all patient subgroups in this trial benefited from postoperative WBI.
The UK Coordinating Committee on Cancer Research trial included 1,030 patients with DCIS or microinvasive disease (invasive disease measuring less than 1 mm) [16]. Patients were randomized to postoperative radiation therapy or observation following margin-negative tumor excision. Some patients in each group received adjuvant tamoxifen therapy. Patients randomized to postoperative radiation therapy received 50 Gy to the whole breast without a boost to the tumor bed. At a median follow-up time of 4.8 years, the incidence of recurrence in the ipsilateral breast was significantly reduced in the patients randomized to postoperative radiation therapy (6 % vs. 14 %, P < 0.001). Although tamoxifen use was not associated with a reduced risk of ipsilateral invasive disease, it was associated with a reduced risk of ipsilateral DCIS recurrence.
In the SweDCIS trial, 1,046 women were randomized to postoperative radiation therapy or observation [17]. Patients randomized to postoperative irradiation had a 5-year incidence of ipsilateral recurrence of 7 %, compared to 22 % in the observation group (P < 0.0001). No difference was seen in OS.
Despite these data from prospective, randomized trials supporting the benefit of postoperative radiation therapy following margin-negative tumor excision, some investigators have supported excision alone for DCIS because of the lack of OS benefit from postoperative radiation therapy. Thus, patients who are unlikely to benefit from postoperative radiation therapy may be selected for breast-conserving surgery only. The MD Anderson Cancer Center selection criteria for breast-conserving surgery alone have been discussed earlier in this chapter.
Limited data exist to support the use of accelerated partial breast irradiation (APBI) for patients with DCIS. APBI is administered two times daily over 5 days. A variety of methods exist for administration of APBI, including the use of balloon catheters or interstitial multicatheter brachytherapy devices and 3-dimensional conformal external beam radiation therapy. The published consensus statement from the American Society for Radiation Oncology (ASTRO) categorizes patients aged 50 years or older with DCIS measuring 3 cm or less in the “cautionary” group for APBI use; patients younger than 50 years of age and those with DCIS larger than 3 cm are considered to be “unsuitable” for APBI [23]. The ASTRO task force asserted that the paucity of data on the use of APBI in patients with DCIS has resulted in uncertainty regarding its use. The ASTRO guidelines encouraged enrollment of patients with DCIS measuring less than 3 cm in the RTOG 04-13/NSABP B-39 clinical trial. This clinical trial was opened in March 2005 and has recently completed accrual. The goal of this trial is to examine the efficacy of APBI modalities compared to each other as well as to WBI.
Adjuvant Tamoxifen
Results from studies to date indicate that following counseling regarding the risks and benefits of tamoxifen therapy, women with estrogen receptor (ER)-positive DCIS without contraindications to tamoxifen therapy should be offered adjuvant tamoxifen for a duration of 5 years.
The NSABP B-24 trial demonstrated a significant reduction in ipsilateral tumor events with adjuvant tamoxifen therapy for patients with DCIS [24]. This trial included 1,804 women with DCIS regardless of ER status. Women were randomized to BCT with tamoxifen or BCT without tamoxifen. At a median follow-up time of 74 months, the rate of breast cancer events was lower in the tamoxifen group (8.2 % vs. 13.4 %, P = 0.0009).
Allred and colleagues retrospectively evaluated 41 % of patients with DCIS in the NSABP B-24 trial to determine the relationship between DCIS ER status and the effects of tamoxifen [25]. In this study, 76 % of women had DCIS that was ER positive. Patients with ER-positive DCIS had a greater reduction in ipsilateral breast tumor recurrence with tamoxifen than patients with ER-negative DCIS (11 % vs. 5.2 %, P < 0.001).
Management of Early-Stage Breast Cancer
Early-stage (stage I and II) breast cancer may be managed successfully with either BCT or mastectomy.
Key Clinical Trials
Trials Comparing BCT and Mastectomy
The NSABP B-06 trial established the survival equivalence of BCT and mastectomy for patients with early-stage breast cancer [6]. This trial compared lumpectomy and axillary lymph node dissection (ALND) either with or without WBI to modified radical mastectomy in patients with a tumor size of 4 cm or less and either N0 or N1 nodal status. A total of 2,163 patients were randomized. No difference was noted between the treatment groups in disease-free survival (DFS) or OS. This was maintained at 20 years of follow-up [26]. Notably, there were significant differences in the local control rates. Patients treated with lumpectomy without WBI had an in-breast recurrence rate of 39.2 %, those treated with lumpectomy with WBI had an in-breast recurrence rate of 14.3 %, and those treated with mastectomy had a chest wall recurrence rate of 10.2 %. In addition to the NSABP B-06 trial, five other randomized trials have demonstrated no difference in DFS and OS between BCT and mastectomy for patients with early-stage disease [7–11].
Axillary Staging
Axillary lymph node status remains the most important prognostic factor for women with operable breast cancer. Much like the treatment of the primary breast tumor, staging and treatment of the axilla has become less invasive over the past several decades. Historically, ALND was required for axillary staging. However, randomized trials evaluating less invasive techniques for operable breast cancer demonstrated that elective ALND had no survival benefit over ALND performed in a delayed fashion once clinically palpable axillary disease became evident [26, 27]. The routine use of ALND for staging of the axilla overtreats the 75 % percent of women with operable breast cancer in whom the axillary lymph nodes are histologically negative. These findings prompted the development of lymphatic mapping and SLNB for breast cancer patients with a clinically negative axilla [28].
In 1991, Giuliano and colleagues initiated a pilot study to examine the use of SLNB for patients with breast cancer. Of the 174 patients enrolled, 114 (65.5 %) had a SLN successfully identified. In 109 of these 114 patients (95.6 %), the status of the SLN accurately predicted the status of the axilla. The results of this pilot study, reported in 1994, revolutionized axillary surgery. Today, SLNB is recognized as a minimally invasive and accurate technique to stage the axilla with the advantage of decreased morbidity [28, 29].
The NSABP B-32 trial compared clinically node-negative patients undergoing SLNB followed by ALND with patients undergoing SLNB with ALND only if a SLN was positive for metastatic disease [30]. A total of 5,611 patients were randomized. The SLN identification rate was 97 %, and the false-negative rate was 9.7 %. Twenty-six percent of patients in the trial had positive SLNs. Over 60 % of patients with metastatic disease in the SLNs had no further positive lymph nodes within the ALND specimen. The NSABP B-32 clinical trial and other randomized trials demonstrated no difference in DFS, OS, and local-regional control rates between patients with negative SLNs who underwent SLNB alone and those who underwent ALND [31, 32]. In addition, patients who undergo SLNB alone have been noted to have decreased morbidity and improved quality of life compared to patients who undergo ALND [32, 33].
The American College of Surgeons Oncology Group (ACOSOG) Z0011 trial evaluated the utility of ALND in patients with clinical T1-2, N0 breast cancer with one or two positive SLNs for whom BCT with WBI was planned [34]. Patients were not eligible if they received neoadjuvant chemotherapy or neoadjuvant hormonal therapy or if their treatment plan included mastectomy, lumpectomy without radiation, or lumpectomy with alternative forms of radiation delivery such as APBI. WBI was administered using standard tangential fields without additional fields. Patients with one or two positive SLNs were randomized to completion ALND or no further surgery. Decisions regarding adjuvant therapy were left to the treating clinicians. The primary endpoint was OS, and the secondary endpoint was local-regional recurrence. After a median follow-up time of over 6 years, no difference was noted between patients randomized to completion ALND and those randomized to no further surgery in terms of OS (91.9 and 92.5 %, respectively; P = 0.25) or DFS (82.2 and 83.8 %, respectively; P = 0.14).
Data from the ACOSOG Z0011 trial also demonstrated that patients randomized to SLNB alone were less likely to have adverse effects than were patients randomized to completion ALND (25 % vs. 70 %, P ≤ 0.001) [35]. Patients in the SLNB-alone group were less likely to have wound infections (3 % vs. 8 %, P ≤ 0.0016), seromas (6 % vs. 14 %, P ≤ 0.0001), paresthesias (9 % vs. 39 %, P < 0.0001), and subjectively reported lymphedema (2 % vs. 13 %, P < 0.0001).
Prior to the reporting of the ACOSOG Z0011 data, completion ALND was the standard of care for patients with metastatic disease identified within SLNs. Following publication of the ACOSOG Z0011 trial, the National Comprehensive Cancer Network (NCCN) added a footnote to its published breast cancer guidelines stating that there was no OS difference for patients with one or two positive SLNs treated with BCT who underwent completion ALND and those who underwent no further surgery [36]. In addition, the American Society of Breast Surgeons issued a consensus statement that supported the omission of completion ALND for patients who meet the ACOSOG Z0011 criteria [37]. The results of the ACOSOG Z0011 trial have revolutionized treatment of the axilla in selected patients with axillary metastasis.
The International Breast Cancer Study Group (IBCSG) 23-01 trial had a design similar to that of the ACOSOG Z0011 trial [38]. In the IBCSG 23-01 trial, patients with micrometastatic disease within the SLN were randomized to ALND versus no further surgery. Unlike the ACOSOG Z0011 trial, the IBCSG 23-01 trial did not exclude patients undergoing mastectomy. Approximately 9 % of patients in each arm of the trial were treated with mastectomy. The investigators recently published the results and showed no differences in OS or local-regional recurrence between the study arms [39].
Recently, the ACOSOG Z1071 trial examined the role of SLNB in patients who presented with N1-2 nodal disease and received neoadjuvant chemotherapy [40]. This trial included patients with clinical T1-4, N1-2 breast cancer who received neoadjuvant chemotherapy. All patients underwent SLNB followed by completion ALND. Complete resolution of axillary disease was noted in 40 % of patients. SLNB identified the nodal status correctly in 84 % of patients; the false-negative rate was 12.4 %. Although this false-negative rate was higher than the predefined acceptable rate of 10 %, removal of two or more SLNs at the time of SLNB reduced the false-negative rate. The results of this trial were recently published in the Journal of the American Medical Association. This trial may significantly impact treatment of the axilla in patients with axillary nodal disease at presentation in whom axillary disease resolves following neoadjuvant chemotherapy.
Selection of Surgical Therapy
BCT or Mastectomy
Selection of therapy for patients with early-stage breast cancer depends on a variety of tumor and patient factors, including the ratio of tumor size to breast size, the presence of multicentric disease, whether the patient can tolerate radiation therapy, and patient preference. Patients with a large tumor in relation to the size of the breast may not achieve an adequate cosmetic outcome after BCT and may be better served by mastectomy. BCT is typically reserved for patients with a tumor size of 4 cm or less. However, BCT with a good cosmetic outcome may also be achievable in women with larger tumors and relatively large breasts. Patients with larger tumors who wish to pursue BCT may be candidates for either neoadjuvant chemotherapy or neoadjuvant hormonal therapy to decrease the tumor size and thus permit BCT. In addition, patients with larger tumors who opt for BCT may be candidates for local tissue rearrangement or placement of myocutaneous tissue flaps to repair the defect resulting from BCT. Patients with multicentric disease are better served by mastectomy as they are considered to have an increased risk of recurrence after BCT.
It is also important to recognize that BCT requires adjuvant radiation therapy. Thus, patients for whom BCT is planned should be evaluated by a radiation oncologist if they have undergone prior irradiation of the breast or a region close to the breast or have a collagen vascular disease. In addition, patients for whom BCT is planned must be willing and able to attend all planned radiation therapy appointments.
Breast Reconstruction After Mastectomy
Mastectomy for early-stage breast cancer may be performed either with or without breast reconstruction. Many patients with early-stage breast cancer who undergo mastectomy are candidates for breast reconstruction.
For many patients, reconstruction can be performed immediately at the time of mastectomy. Immediate reconstruction allows for skin-sparing mastectomy which preserves the patient’s own skin, thus optimizing cosmetic outcomes. Highly selected women with early-stage breast cancer may be candidates for immediate reconstruction with preservation of the nipple-areolar complex. Eligibility for this procedure has been described previously in this chapter. Patients for whom adjuvant radiation therapy is planned are not ideal candidates for nipple-areolar-complex-sparing mastectomy because of the effects of radiation on the preserved nipple. In addition to providing improved cosmesis resulting from preservation of the skin and/or the nipple-areolar complex, immediate reconstruction provides a psychological benefit for the patient. Patients undergoing immediate reconstruction also benefit from completing therapy and reconstruction in one surgery.
If no postoperative radiation therapy is planned, patients may have immediate reconstruction performed using implants or autologous tissue; tissue flaps that can be used include the transverse rectus abdominis myocutaneous flap, deep inferior epigastric perforator flap, latissimus dorsi flap with an implant, and other tissue flaps. However, if adjuvant radiation therapy may be required, a tissue expander should be placed. A tissue expander allows for preservation of the skin at the time of mastectomy, and the expander can be deflated at the time of radiation therapy to permit adequate irradiation of the chest wall and regional nodal basins. Removal of the tissue expander and reconstruction with either an implant or autologous tissue takes place approximately 1 year after completion of radiation therapy.
Axillary Staging
Axillary staging is required for all patients with early-stage breast cancer. Information about the axillary nodal status is valuable prognostic information and assists in tailoring adjuvant therapies. For example, for patients with small tumors without lymph node involvement, adjuvant chemotherapy may not be recommended; however, detection of lymph node involvement in a patient with a small tumor would prompt a recommendation for chemotherapy. In addition, detection of axillary lymph node involvement in a patient younger than 40 years or more than four involved axillary lymph nodes in any patient would prompt a recommendation for adjuvant radiation therapy in patients treated with mastectomy, whereas in the absence of nodal metastases, postmastectomy radiation therapy (PMRT) would not be recommended.
Thus, patients with clinically node-negative breast cancer should undergo SLNB for staging of the axilla. Patients with a positive SLN should be appropriately selected for completion ALND versus no further surgery according to the principles outlined previously.
At MD Anderson, patients for whom BCT with WBI is planned and who meet the eligibility criteria used in the ACOSOG Z0011 trial undergo intraoperative lymphatic mapping with SLNB at the time of segmental mastectomy. At the time of SLNB, the SLNs are sent to the pathology department for permanent-section examination. Patients with one or two positive SLNs who have negative tumor margins proceed to adjuvant systemic therapy and WBI with no further surgery.
The current MD Anderson practice regarding completion ALND was established during a multidisciplinary conference held to discuss the results of the ACOSOG Z0011 trial and apply these results safely to patients [41]. This conference included clinicians from the Departments of Surgical Oncology, Radiation Oncology, Breast Medical Oncology, Diagnostic Radiology, and Pathology. The participants reached a consensus that omission of completion ALND was appropriate for patients with clinical T1-2, N0 breast cancer and one or two positive SLNs expected to undergo BCT with WBI but not for patients expected to undergo mastectomy or APBI or for patients who underwent neoadjuvant chemotherapy or neoadjuvant hormonal therapy. Special consideration was given to patients with lobular histology as patients with lobular carcinoma were underrepresented in the ACOSOG Z0011 trial and small-volume axillary disease may be of clinical relevance in patients with lobular histology. Both of these factors should be taken into consideration when patients with lobular histology are counseled about completion ALND. Hormone receptor status is also an important consideration as 83 % of ACOSOG Z0011 participants had ER-positive disease. Although ER status was not significantly associated with local-regional recurrence on multivariable analysis, at MD Anderson, hormone receptor status is considered within a broad context of factors when patients are counseled about completion ALND. Age is another important factor to consider. More than 62 % of patients in each arm of the ACOSOG Z0011 trial were older than 50 years. In addition, age younger than 50 years was a significant predictor of local-regional recurrence on multivariable analysis. Thus, patients younger than 50 years should be carefully counseled regarding completion ALND. Nodal burden may also play an important role in risk determination. At MD Anderson, a nomogram that incorporates the size of SLN metastases and the ratio of positive to negative nodes harvested at SLNB may be used to counsel patients regarding the need for completion ALND [42]. At MD Anderson, patients with a positive SLNB expected to undergo mastectomy and those expected to undergo BCT with alternative forms of radiation therapy undergo completion ALND.
Surgical Techniques
Breast-Conserving Surgery
Patients undergoing breast-conserving surgery for nonpalpable early-stage breast cancer require image-guided localization of the tumor.
Incision placement is key to achieving optimal cosmetic outcomes. The tumor is excised with a rim of normal breast tissue. The specimen is then oriented and sent to the pathology department, where it is imaged with specimen radiography, inked, sectioned, and reimaged. If close margins are identified on specimen radiography, re-excision is performed, and the excised tissue is sent to the pathology department for permanent-section examination. The border of the surgical cavity is marked with radiopaque clips to facilitate radiation therapy planning.
Patients with larger defects after tumor excision may benefit from involvement of a plastic surgeon for local tissue rearrangement (Fig. 17.4a) or reconstruction using a latissimus dorsi flap (Fig. 17.4b). If necessary, a procedure may be performed on the contralateral breast to achieve symmetry, either during the same surgery when the tumor is excised or following completion of radiation therapy at a second surgery.
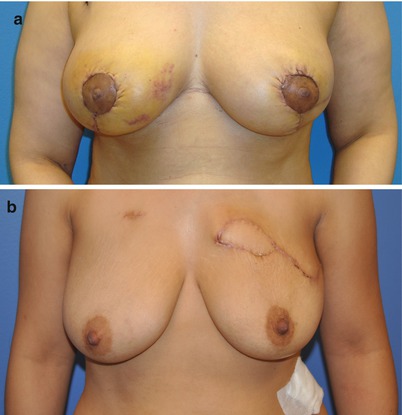

Fig. 17.4
(a) Cosmetic outcome in a patient requiring re-excision for margin control with local tissue rearrangement and contralateral symmetry procedure. (b) Breast-conserving surgery with repair of the partial mastectomy defect using a latissimus dorsi flap for volume replacement (Photos courtesy of Dr. David M. Adelman)
The findings on the final pathology review dictate whether additional surgical therapy will be needed. As described previously, at MD Anderson, a margin of less than 2 mm prompts consideration for a return to the operating room for re-excision. If negative margins cannot be achieved after multiple re-excisions, mastectomy is indicated.
Mastectomy
Surgical options for patients undergoing mastectomy for early-stage breast cancer include total mastectomy, skin-sparing mastectomy, and, for some highly selected patients, nipple-areolar-complex-sparing mastectomy.
Regardless of the type of mastectomy, intraoperative specimen radiography is performed to determine the adequacy of margins. Excision of additional skin may be necessary if superficial disease is identified.
As discussed previously, careful selection of patients for nipple-areolar-complex-sparing mastectomy is crucial to optimize outcomes. If there is suspicion of disease beneath the nipple or areola, intraoperative assessment of the tissue underlying the circumference of the areolar margin at 3, 6, 9, and 12 o’clock as well as directly underlying the nipple may be performed by the pathologist using frozen section examination. The nipple-areolar complex should be excised if malignant cells are identified on frozen section examination.
Patients undergoing skin-sparing mastectomy or nipple-areolar-complex-sparing mastectomy undergo initiation of reconstruction with placement of a tissue expander. If the likelihood of adjuvant radiation therapy is very small, immediate reconstruction can be performed using an implant or a myocutaneous flap.
Axillary Lymph Node Staging
In patients with a clinically negative axilla, axillary staging should be performed with SLNB. SLNB requires lymphatic mapping, which can be accomplished with blue dye or a radioactive tracer, and SLN dissection. Some surgeons choose to have patients undergo preoperative lymphoscintigraphy as well to identify patterns of lymphatic drainage.
For patients undergoing preoperative lymphoscintigraphy, lymphoscintigraphy is most often performed with injection of high-dose technetium-labeled sulfur colloid (2.5 mCi) on the day prior to surgery. The technetium-labeled sulfur colloid can be injected peritumorally or under the areola. Patients with nonpalpable tumors require imaging guidance for peritumoral injection. Peritumoral injection has the advantage of identifying drainage patterns of the tumor outside of the axilla, such as drainage to the internal mammary lymph nodes. Lymphoscintigraphy is performed 15–30 min following radiocolloid injection and then at 30- to 60-min intervals thereafter until drainage to the SLN is identified. The inability of lymphoscintigraphy to identify a SLN on the day before surgery does not necessarily indicate failure of mapping; in some patients, drainage to SLNs will occur, and a SLN will be identified with a handheld gamma probe at the time of surgery. However, if drainage is not identified on lymphoscintigraphy performed the day before surgery, consideration should be given to reinjection of low-dose technetium-labeled sulfur colloid on the day of surgery.
On the day of surgery, patients injected the day before surgery with high-dose technetium-labeled sulfur colloid are taken directly to the operating room. Patients who did not undergo injection of high-dose technetium-labeled sulfur colloid the day before surgery should be injected with a low dose (0.5–1 mCi) of technetium-labeled sulfur colloid 1–4 h before they are taken to the operating room. If dual-modality SLN mapping is planned (i.e., use of both blue dye and radiotracer), prophylaxis for allergic reactions to the blue dye solution should be administered intravenously in the operating room. This prophylaxis includes diphenhydramine, steroids, and famotidine. Five milliliters of lymphazurin blue dye should be injected peritumorally for patients undergoing breast-conserving surgery or either peritumorally or under the areola for patients undergoing mastectomy. The breast should be massaged for 5 min to facilitate lymphatic drainage. A handheld gamma probe is used to transcutaneously localize the SLN within the axilla. A transverse incision is made close to the transcutaneously identified node along the standard ALND incision line below the axillary hairline. The gamma probe may be utilized to guide the dissection. Alternatively, blue-stained lymphatics may be used to guide the dissection. SLNs are defined as blue-stained lymph nodes and lymph nodes containing radioactivity as identified by the gamma probe.
Patients in whom mapping is more likely to fail to identify a SLN include patients who have undergone prior breast surgery, patients over 70 years of age, and obese patients. Patients who do not have a SLN identified should undergo ALND. The technique for ALND is described later in this chapter.
Radiation Therapy
The Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) has examined all of the randomized trials where breast conservation was performed with or without radiation therapy [43]. At 15 years of follow-up, the absolute reduction in mortality with radiation therapy after breast-conserving surgery was 5.1 % in node-negative patients and 7.1 % in node-positive patients. These data suggest that the addition of radiation not only improves local control but also improves survival.
Two randomized trials have suggested that in selected older patients with small, low-grade tumors, breast-conserving surgery without radiation therapy may be appropriate [44, 45]. The Cancer and Leukemia Group B (CALGB) C9343 trial included women over 70 years of age with T1N0 breast cancer and randomized them to breast-conserving surgery with or without radiation therapy. All women, 97 % of whom had ER-positive tumors, were treated with adjuvant tamoxifen. No differences in DFS and OS were seen although the local recurrence rate was lower in patients randomized to radiation (1 % vs. 4 %, P < 0.001). The Canadian trial was similar to the CALGB C9343 trial. Although the Canadian trial was open to women 50 years of age and older, the mean age was 68 years, and 80 % of women had ER-positive tumors. At a median follow-up time of 5.6 years, no difference was seen in DFS or OS although the local recurrence rate was lower in patients randomized to radiation (0.6 % vs. 7.7 %, P < 0.001). Generally, patients with early-stage breast cancer selected for breast-conserving surgery without radiation include women 70 years of age or older with an expected survival of less than 10 years with T1, N0, ER-positive breast cancer.
APBI is an option for carefully selected patients with early-stage breast cancer. A variety of methods exists for administration of APBI as have been described previously in this chapter. Proponents of APBI argue that the majority of breast cancer recurrences occur in or adjacent to the tumor bed; the abbreviated course of treatment may increase the feasibility of BCT for many women; and the abbreviated course of treatment may improve radiation therapy compliance. The previously discussed RTOG 04-13/NSABP B-39 trial, which directly compares WBI to APBI in early-stage breast cancer, will provide data on local recurrence and survival and assess differences in outcomes between the two radiation treatment strategies.
While the results of this trial are awaited, a consensus statement from ASTRO was developed to guide the use of APBI outside of the context of a clinical trial [23]. According to the consensus statement, patients suitable for APBI include patients 60 years of age or older with a unifocal, T1, ER-positive tumor with no lymphovascular invasion and resection margins of at least 2 mm. Patients for whom ASTRO was not certain about the appropriateness of APBI include patients with invasive lobular histology, a tumor size of 2.1 cm to 3 cm, ER-negative disease, focal lymphovascular invasion, or margins less than 2 mm. Patients considered unsuitable for APBI include those with T3 or T4 disease, ER-negative disease, multifocality, multicentricity, extensive LVI, or positive margins.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree