T
Primary tumor
Tx
Primary tumor cannot be assessed
To
Primary tumor cannot be detected
Tis
aDCIS, LCIS, Paget disease of the nipple with no tumor
T1
Tumor ≤ 2 cm in the greatest dimension
T1mic
Microinvasion ≤0.1 cm in the greatest dimension
T1a
Tumor >0.1 cm but ≤0.5 cm in the greatest dimension
T1b
Tumor >0.5 cm but ≤1 cm in the greatest dimension
T1c
Tumor >1 cm but ≤2 cm in the greatest dimension
T2
Tumor > 2 cm but ≤5 cm in the greatest dimension
T3
Tumor > 5 cm in the greatest dimension
T4
Tumor of any size with direct extension to chest wall or skin
T4a
Extension to the chest wall, but not pectoralis muscle
T4b
Edema (including peau d’orange or ulceration of the skin of the breast or satellite skin nodules confined to the same breast)
T4c
Both T4a and 4b
T4d
Inflammatory carcinoma
N
Regional lymph nodes
Nx
Regional lymph nodes cannot be assessed
N0
No regional lymph node metastasis
N1
Micrometastasis
Metastases in 1–3 axillary lymph nodes ± internal mammary nodes
N2
Metastases in 10 or more axillary lymph nodes
Infraclavicular lymph nodes (level III)
Clinically detected internal mammary lymph nodes
>3 axillary lymph nodes and internal mammary nodes
Ipsilateral supraclavicular lymph nodes
N3
Metastases in 10 or more axillary lymph nodes
Metastases to infraclavicular lymph nodes (level III)
M
Distant metastases
M0
No distant metastases
cM0 (i+)
Circulating tumor cells or microscopic tumor cells in the bone marrow
No clinical or radiological distant metastasis
M1
Distant metastases
Table 15.2
AJCC stage grouping classification of breast cancer
Stage grouping | |
|---|---|
Stage 0 | Tis N0 M0 |
Stage I | T1 N0 M0 |
Stage IIA | T0 N1 M0 |
T1* N1 M0 | |
T2 N0 M0 | |
Stage IIB | T2 N1 M0 |
T3 N0 M0 | |
Stage IIIA | T0 N2 M0 |
T1 * N2 M0 | |
T2 N2 M0 | |
T3 N1 M0 | |
T3 N2 M0 | |
Stage IIIB | T4 N0 M0 |
T4 N1 M0 | |
T4 N2 M0 | |
Stage IIIC | Any T N3 M0 |
Stage IV | Any T Any N M1 |
The prognostic factors to estimate the chance of recurrent disease and distant metastases include tumor size, histological grade, and lymph node status. Breast cancer cells can spread via the lymphatic system to the regional lymph nodes, involving the low axillary lymph nodes (level I) first followed by the mid- (level II) and high axillary lymph nodes (level III) (Fig. 15.1). Level I lymph nodes are lateral to the lateral border of the pectoralis minor muscle. Level II lymph nodes are between the medial and lateral borders of the pectoralis minor muscle and also include interpectoral lymph nodes (Rotter’s nodes). Level III lymph nodes are nodes medial to the medial margin of the pectoralis minor muscle and inferior to the clavicle and are not commonly resected due to an increased risk of lymphedema. The standard approach to staging lymph nodes via axillary nodal dissection often involves removal of the low and mid-axillary lymph nodes. 12–25 % of breast cancer patients have internal mammary drainage with approximately 9–30 % with internal mammary nodal metastases, often those with large and deep medially located breast cancer, who can have a 5-year survival of 95 % after appropriate therapy [4, 5]. The seventh edition of the TNM staging manual considers internal mammary lymph nodes to be axillary nodes for the purposes of staging (cN2b or cN2c). If supraclavicular nodes are involved, then this is designated as N3 disease with poorer prognosis, because patients with infraclavicular and supraclavicular nodal disease tend to have increased tumor burden compared with those with only axillary nodal disease.
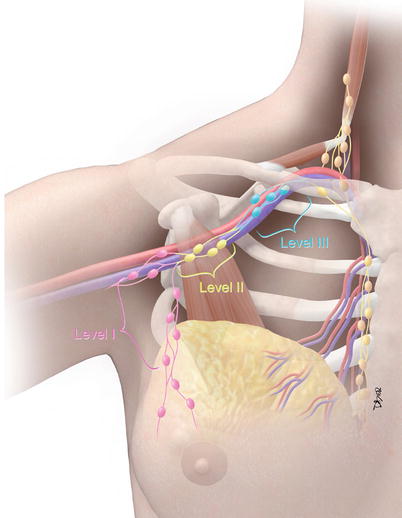

Fig. 15.1
Regional lymph node staging in breast cancer. Level I lymph nodes (pink color): nodes lateral to the pectoralis minor muscle (brown muscle). Level II lymph nodes (yellow color): nodes between the medial and lateral margins and posterior to the pectoralis minor muscle. Rotter’s nodes are located between the pectoralis major and minor muscles. Level III lymph nodes (sky blue color): nodes medial to the pectoralis minor muscle and inferior to the clavicle (Courtesy of David Bier, Medical Illustrator, The University of Texas MD Anderson Cancer Center)
The National Comprehensive Cancer Network (NCCN) Guidelines for Breast Cancer are statements and consensus from experts regarding current acceptable approaches to the treatment of breast disease. The use of imaging in the staging evaluation of breast disease is also included. For example, the latest version (3.2013) of the NCCN guidelines includes breast MRI as an optional imaging work-up added for the ductal carcinoma in situ (DCIS) staging evaluation. For invasive breast cancer, the uses of optional breast MRI for mammographically occult tumors, bone scan, or sodium fluoride PET/CT for clinical stage IIIA were added or modified [6].
Local Staging of Breast Cancer by Imaging
Mammography
Diagnostic mammography or problem-solving mammography is performed when a patient presents with a palpable finding or when a suspicious finding is detected with screening mammography. A radiopaque BB or skin marker may be placed directly over the suspicious region before the mammogram is obtained. Many facilities have adopted a filmless practice and have successfully converted from conventional film mammography to digital mammography. For both conventional and digital mammography, a standard examination consists of a mediolateral oblique and a craniocaudal view for each breast. Additional views may be indicated to properly evaluate the abnormality. These additional views include a 90° lateral view to triangulate the abnormality and spot compression views, in craniocaudal and lateral projections, and to evaluate a possible mass, asymmetric density, or superimposition of normal breast parenchyma. Magnification views, commonly performed in craniocaudal and lateral projections, facilitate characterization of microcalcifications. With advances in digital acquisition and processing technology, digital tomosynthesis is being incorporated into mammography systems and may provide a solution to the problem of distinguishing overlapping structures in the breast, increasing the sensitivity and specificity of mammography for cancer detection and diagnosis [7]. Clinical trials are currently under way to establish the efficacy of digital breast tomosynthesis and to define its role in future practice.
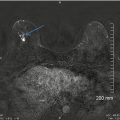
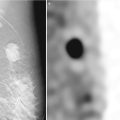
In contrast to a screening mammography examination, a diagnostic mammogram can also provide valuable information during the staging work-up of a newly diagnosed breast cancer. If a patient has DCIS with associated microcalcifications, then magnification views are needed to determine the extent of the microcalcifications, the multifocal or multicentric distribution, and the proximity of the calcifications to the nipple. The accuracy of this information is very important because this disease has a favorable prognosis if there is no progression to invasive carcinoma, and the treatment options for DCIS include breast-conserving surgery with radiation therapy or mastectomy. Accurate size measurement of the area of involvement can help clinicians determine the best surgical approach. In general, an axillary lymph node dissection is not necessary, but a sentinel lymph node biopsy would be performed. If noncontiguous groups of suspicious microcalcifications are identified during the staging evaluation, then mammographic-guided breast interventions such as stereotactic-guided core biopsy using vacuum-assisted devices would be suggested. Prior to surgery, mammographic-guided needle localization of multiple sites or bracketing of a large area is commonly performed to assist surgeons in obtaining negative margins at surgery (Fig. 15.2). Immediately after resection of the targeted lesion, the biopsy or surgically resected specimen can be imaged while the patient is still under anesthesia in order to verify the margins. This “specimen radiography” is very helpful, not only to optimize the chance of obtaining final negative margins but also to provide additional information in patients who had a complete response to preoperative chemotherapy, as the only image-detectable finding is often the biopsy clip placed at the time of the initial biopsy or residual calcifications (Fig. 15.3). Radioactive seed localization is being incorporated into several practices, replacing wire localization prior to surgery, due to several advantages. These advantages include precise knowledge of the tumor with the radioactive seed, allowing the surgeon to determine the incisional site. Also, the radiologist’s localization approach can be independent of the surgeon’s incisional approach, and the seed(s) can be placed days prior to surgery [8].
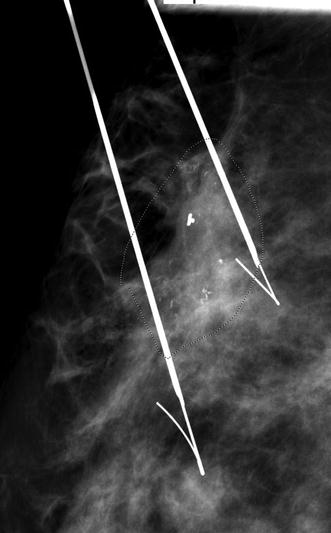
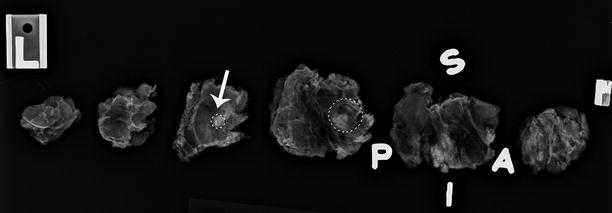

Fig. 15.2
Preoperative needle localization. A 57-year-old female with a diagnosis of ductal carcinoma in situ (DCIS) of the breast. The 3 cm area of pleomorphic calcifications (area demarcated with faint doted circle) was bracketed on the day of surgery using two Kopans localization needles. Final pathology confirmed a 3.5 cm area of DCIS successfully resected with final negative surgical margins

Fig. 15.3
Specimen x-ray. A 56-year-old female with invasive ductal carcinoma of the breast who received segmentectomy after completion of neoadjuvant chemotherapy. The biopsy clip: and the doted circles (white arrow and the doted circles) visible on the specimen radiograph confirmed the site of biopsy-proven carcinoma. Final pathology revealed no residual tumor. S superior margin, P posterior margin, I inferior margin, A anterior margin
Breast Ultrasound
Primary Breast Site
Breast ultrasound is commonly used as a diagnostic tool for characterization of lesions detected via mammography or clinical examination and for staging of newly diagnosed breast cancer. A breast ultrasound examination can determine the size of the index breast carcinoma; determine the unifocal, multifocal, or multicentric status of the known carcinoma; and evaluate associated lymph node involvement. A linear array transducer should be used to perform the examination utilizing the highest center frequency (7.5–18-MHz probe) possible in order to provide high-resolution images of the breast. However, penetration to the chest wall is needed. Therefore, many facilities have a second transducer with a lower frequency (2–5-MHz probe) for better penetration to the chest wall, especially in large breasts with deep lesion(s). Images of the lesion in both longitudinal and transverse planes or radial and orthogonal antiradial planes are commonly used to characterize the findings, in order to obtain measurements in two perpendicular planes. For reporting of the findings, the BI-RADS risk assessment categories are used and should be adhered to [9]. At our facility, the entire ipsilateral breast and the regional nodal basins are scanned to determine the full extent of the index lesion, any associated satellite or synchronous lesions, and associated nodal disease.
Breast tumors are usually hypoechoic solid masses, with irregular margins, posterior acoustic shadowing, and internal vascularity on color Doppler imaging (Fig. 15.4). Unlike invasive ductal carcinoma, invasive lobular carcinoma does not tend to form a dominant or palpable mass but presents as vague architectural distortion or focal asymmetry on mammography [10, 11]. This is likely due to the microscopic single-file growing pattern of the tumor cells and their infiltrative nature, which result in high false-negative rates on mammography. US has been reported to have higher sensitivity in the detection of invasive lobular carcinoma, with the most common imaging feature being a hypoechoic mass or masses with posterior acoustic shadowing, in an infiltrative pattern [12–16].
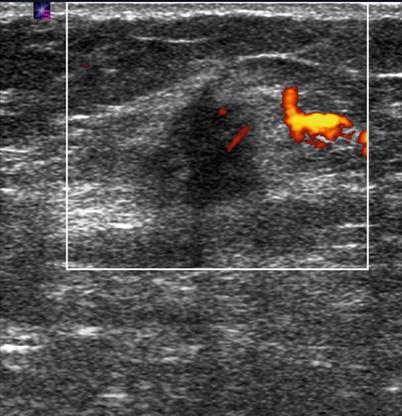

Fig. 15.4
Ultrasound of breast carcinoma. Power Doppler ultrasound of the right breast (white box) in a 58-year-old female showing a 1.6 cm hypoechoic solid mass with posterior shadowing and internal vascularity, which was biopsy-proven carcinoma
Most invasive carcinomas have a mixture of invasive and noninvasive or intraductal components. The intraductal components or DCIS may present as linear hypoechoic long ductal extensions with an extensive branching pattern from the index mass towards the nipple, or linear extensions between hypoechoic masses. Sometimes the calcifications associated with DCIS can be detected on ultrasound as smaller hyperechoic foci within distended ducts or within a suspicious mass [17]. Some DCIS present as intraductal or intracystic masses [18]. In addition, US can be used as an adjuvant modality to detect any associated invasive carcinoma in patients with newly diagnosed DCIS, and ultrasound-guided biopsy is performed. For multicentric disease in which the lesions are more than 5 cm apart or the multiple tumor foci lie in different quadrants of the breast, extended field of view is commonly used in some facilities (Fig. 15.5). This technology allows the radiologist to present a wider field of view than is available using standard real-time transducers. In addition to imaging multiple lesions on one image, the extended field of view also enables the operator to provide an image documenting the distance of the index mass from the nipple (Fig. 15.6).
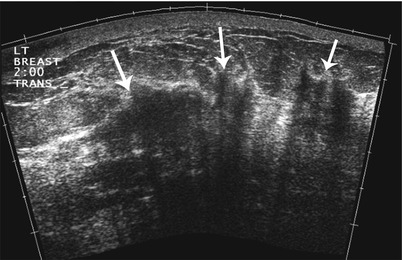
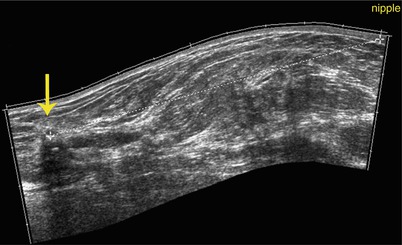

Fig. 15.5
Extended-field-of-view ultrasound. A 43-year-old female with multicentric invasive ductal breast carcinoma. Extended-field-of-view ultrasound imaging of the left breast demonstrates multiple areas (arrows) of heterogeneous shadowing tissue and distortion of the normal tissue. Biopsy confirmed invasive ductal carcinoma at 2 sites and final pathology at mastectomy showed a 9 × 5 cm area of tumor

Fig. 15.6
Ultrasound: distance from nipple. Extended-field-of-view ultrasound imaging of the right breast demonstrates how the measurement of the distance from the hypoechoic irregular carcinoma (yellow arrow) to the nipple is measured
Regional Lymph Nodes
Nodal Staging y Imaging
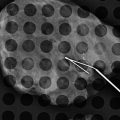
Ultrasound of the nodal basins is being performed at more facilities now than previously, in both academic and private practices. In many places, sonographic evaluation of the ipsilateral axilla is conducted for all newly diagnosed breast cancer cases to assess the size and morphology of any suspicious or abnormal nodes. When a lymph node is infiltrated with tumor, the size of the lymph node is usually increased in addition to the change in the cortical morphology of that node. Abnormal cortical morphology includes eccentric cortical thickening with focal cortical lobulation, or diffuse hypoechoic lymph node with the loss of the central echogenic hilum [19, 20]. The morphology of the lymph node is suggested to be more specific (88.4–98.1 %) than the lymph node size (55.6–97.3 %) [21]. Upon detection of an abnormal lymph node, pathology confirmation of nodal malignancy can be performed prior to surgery by ultrasound-guided core biopsy or fine-needle aspiration biopsy. This information may assist the surgeon in the decision to proceed directly to an axillary lymph node dissection (ALND) as opposed to a sentinel lymph node biopsy (SLND). Radiological marker placement at the time of the ultrasound-guided biopsy can aid in facilitating subsequent confirmation of biopsy-proven metastatic node recovery or in ensuring the complete removal of the breast cancer that has disappeared after the completion of neoadjuvant chemotherapy. At our institution, the infraclavicular and internal mammary nodal basins are also evaluated at the initial staging sonographic examination of all newly diagnosed breast cancer cases (Fig. 15.7a–c).
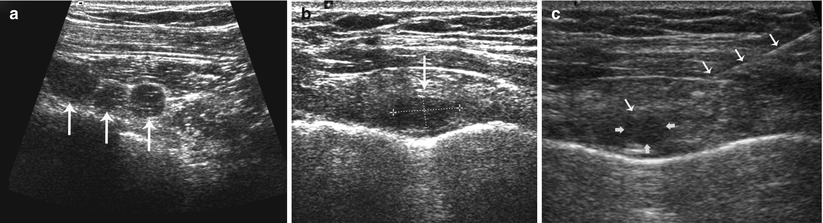

Fig. 15.7
(a–c) Staging ultrasound of the infraclavicular and internal mammary nodal basins. (a) Transverse grayscale ultrasound shows abnormal round hypoechoic infraclavicular lymph nodes (three arrows) without central fatty hila. (b) Transverse grayscale ultrasound shows an abnormal enlarged hypoechoic lymph node (single arrow and doted lines) in the first internal mammary space. (c) Fine-needle aspiration biopsy (white arrows along needle shaft) confirmed metastatic lymph node (yellow arrows)
Nodal Staging by Surgery
Sentinel lymph node dissection (SLND) is based on the concept that the breast has an orderly pattern of lymphatic drainage with specific lymph nodes, or sentinel nodes, that drain the breast first, followed by drainage to the remaining nodal basin. This idea was first reported by Braithwaite over 100 years ago after observing the lymphatic drainage pattern of a gangrenous appendix. The first clinical applications were presented in the 1970s for penile cancer, although the technique did not become widely used because of its difficulty [22]. In the early 1990s, a more facile technique was created for melanoma that allowed for widespread implementation [23]. Previously, women had usually undergone ALND for staging of axillary nodes, with associated morbidities including functional deficits, chronic pain, and development of lymphedema. Unfortunately, many of these patients had no nodal metastases and thus suffered the morbidities without an oncologic benefit. Thus, there was tremendous interest in applying SLND to breast cancer patients, and SLND was quickly validated as an accurate technique for staging nodal basins in breast cancer patients with increased sensitivity and decreased risks [24–26]. There are many variations in the SLND technique, although all involve the basic principle of injecting the breast with a mapping agent or combination of agents (usually radioisotope and blue dye), which is then allowed to drain to the nodal regions and collect in the sentinel node(s). This can be performed preoperatively or intraoperatively. At the time of surgery, any node collecting the mapping agent (as determined by being blue if blue dye is used or being “hot,” i.e., having increased radioactivity by handheld Geiger probe if radioisotope is injected) are removed and sent for pathological evaluation.
In clinically node-negative patients who are undergoing surgery as the first component of their breast cancer treatment, SLND is the standard surgical approach to axillary staging. Multiple studies have demonstrated that an SLN can be identified in 93–99 % of patients with a false-negative rate (i.e., number of patients with axillary metastases in which no cancer is seen in the SLN) of 5–11 % [27, 28]. If the SLN is negative for metastases, then no further axillary surgery is required and the remaining lymph nodes can be left in place. While initially all patients with axillary metastases underwent ALND, this paradigm has shifted since the American College of Surgeons Oncology Group (ACOSOG) Z0011 trial, which demonstrated that ALND can be safely omitted in carefully selected patients with early-stage breast cancer who undergo breast-conservation therapy and whole-breast radiation [7, 29]. ALND is still recommended for patients who do not meet the eligibility criteria for this trial, such as those with large tumors or extensive nodal involvement, those who have received neoadjuvant chemotherapy (NCT), or those undergoing mastectomy [30].
Axillary lymph node dissection is now performed only if there is confirmation of axillary metastases, either by ultrasound and needle biopsy or by SLND. ALND allows for a more thorough staging of the axillary nodes because all nodes are removed and the total number of involved nodes can be counted. In addition to being a diagnostic procedure, there is also a therapeutic advantage to ALND in node-positive women [31]. Standard ALND involves the removal of the level I and II axillary lymph nodes. Level III nodes are not routinely removed unless there is evidence of their involvement. Unfortunately, ALND is associated with significant short- and long-term morbidities. Short-term effects include the need for uncomfortable postoperative drains and the potential for seromas. Ligation of intercostobrachial nerves, which often occurs during the resection, can lead to pain and neuropathies that can be permanent. Functional limitations of arm abduction are common in the immediate postoperative period and may persist even with aggressive physical therapy. Perhaps the most significant effect is the possibility of lymphedema, which requires aggressive and time-consuming therapy with physical therapy, diet modifications, and compression garments and which carries an increased risk of cellulitis [32].
Breast MRI
Technique
A breast MRI is performed with the patient lying in a prone position within a 1.5 T or higher magnetic field strength scanner. Only breast-dedicated multiphase array coils should be used to ensure adequate spatial resolution. The examination usually utilizes bilateral imaging techniques for several reasons, including the ability to assess asymmetric enhancement between the breasts as well as evaluation for contralateral carcinoma in patients with newly diagnosed breast cancer. Common sequences of a standard examination include a precontrast T1-weighted pulse sequence to delineate fat from a lesion or lymph node, a precontrast T2-weighted pulse sequence with fat suppression to separate cysts from most solid masses, a time series of contrast-enhanced T1-weighted sequences to enhance detection of breast masses, or a dynamic contrast-enhanced series. This series usually consists of a minimum of three time points over a 6–8-min period after intravenous administration of the contrast medium. The minimal imaging parameter requirements for the dynamic acquisition as recommended by the American College of Radiology include a slice thickness ≤3 mm and in-plane resolution ≤1 mm to facilitate the evaluation of essential morphological details such as lesion margins, spiculations, and internal enhancement [33]. Interpretation is based on the descriptors from the BI-RADS MRI lexicon [34].
Role of MRI in Breast Cancer Staging
During the last decade, breast MRI was routinely used in the preoperative evaluation of newly diagnosed breast cancer cases for assessing the extent of the primary tumor site and for identifying mammographically or sonographically occult multicentric and contralateral cancers [Fig. 15.8a–c]. In the last few years, this practice has been heavily scrutinized, and the role of MRI in staging remains a source of considerable discussion. MRI can be helpful in defining the extent and size of the primary breast carcinoma and in detecting additional foci of malignancy within the ipsilateral breast in up to 16 % of patients, and MRI can detect malignancies that were occult on mammography within the contralateral breast in up to 4 % of patients [35, 36]. A meta-analysis of 19 studies and prospective randomized trials consisting of 2,610 patients reported that the addition of MRI-detected lesions resulted in more extensive surgery than planned, with conversion from breast-conserving surgery to mastectomy or a larger excision in 19 % of patients [35]. No benefit on the reoperation rate was reported [35, 37]. Initially, there was an assumption that treatment of additional foci of tumor visible only on MRI would result in better clinical and survival outcomes. A retrospective study of 756 women with breast cancer comparing women who had preoperative breast MRI and women who did not have breast MRI reported no significant difference in the local recurrence rate (P = 0.51) after a median follow-up of 4.6 years [38]. Two years later, the COMICE (comparative effectiveness of MRI in breast cancer) multicenter trial from the United Kingdom reported no significant reduction in the reoperation rate in the women who were randomly assigned to receive MRI compared with those who did not receive MRI (P = 0.77) [37]. While these publications suggested that preoperative MRI is not necessarily indicated for every patient, the ability of MRI to detect additional tumor foci and contralateral cancer not detected via physical examination or mammography should not be discounted. Lehman et al. [39] reported a 3.1 % incidence of contralateral malignancy detected by MRI only and not seen with mammography or clinical examination. The effect of preoperative MRI-based decisions on recurrent disease or overall survival remains unclear and is a subject for future clinical research. Breast MRI is still recommended for selected cases of invasive lobular carcinoma or inflammatory breast cancer, to assess tumor involvement in the skin, nipple, and/or chest wall (Fig. 15.9a, b) [40, 41]. Because of the diffuse infiltrative growth pattern of invasive lobular carcinoma, MRI may allow better visualization of the tumor in a background of extensive fibrosis and help facilitate a more effective biopsy and surgical plan. At our institution, all patients who present with adenocarcinoma in the axilla without a diagnosis of a primary carcinoma but with tumor markers raise the suspicion of an occult breast primary tumor, and MRI is recommended (Fig. 15.10a, b). This has been demonstrated to be beneficial in identifying the primary tumor [42]. When a primary breast lesion can be detected, proper staging can be performed, facilitating targeted therapy or consideration for breast conservation therapy in conjunction with radiotherapy, depending on the stage of the carcinoma.