© Springer International Publishing Switzerland 2017
Massimo Loda, Lorelei A. Mucci, Megan L. Mittelstadt, Mieke Van Hemelrijck and Maura Bríd Cotter (eds.)Pathology and Epidemiology of Cancer10.1007/978-3-319-35153-7_11. The Intersection of Epidemiology and Pathology
(1)
Harvard Medical School, 77 Avenue Louis Pasteur, NRB 241A, Boston, MA 02115, USA
(2)
Harvard T.H. Chan School of Public Health, 655 Huntington Avenue, Boston, MA 02115, USA
(3)
Harvard T.H. Chan School of Public Health, 677 Huntington Avenue, Kresge 920a, Boston, MA 02115, USA
(4)
Department of Pathology, Dana Farber Cancer Institute and Brigham & Women’s Hospital, Harvard Medical School, 450 Brookline Avenue, Boston, MA 02115, USA
Edward L. Giovannucci
Lorelei A. Mucci
Keywords
PathologyEpidemiologyPatho-epidemiologyColorectal cancerProstate cancerGlioblastomaBreast cancerHematologic malignanciesBiomarkersEtiology1.1 The Intersection of Pathology and Epidemiology
As is the trend in many disciplines, research groups are approaching cancer using interdisciplinary approaches to gain a more complete understanding of the disease as well as to identify predictive biomarkers at molecular, individual, and population levels [1]. Pathology and epidemiology are central to these ambitions, and work together toward the common goal of elucidating disease etiology and progression. Modern cancer biology research is increasingly focused on identification of genetic heterogeneity via molecular pathology, but it remains important to contextualize individual molecular profiles within the individual’s lifetime environmental exposures (“exposome”), resulting gene: environment interactions, and disease trajectory [2]. Furthermore, population-level trends gathered via epidemiological models can be integrated with molecular pathologic data to elucidate etiology simultaneously at molecular, individual, and population levels. Identification of trends and associations driving cancers on a population level provide opportunity for predictive markers, screening opportunities, and therapeutic advances with a greater impact. It is our hope that in making pathology accessible to epidemiologists, and epidemiology accessible to pathologists, trends that are important in populations of cells and of people might be identified and acted upon with greater frequency.
Beyond this, the two fields work more symbiotically. It is increasingly clear that cancers, such as prostate, colon, or breast cancer, are not single diseases but rather are comprised of many subtypes defined by molecular pathology and histology. Patho-epidemiology incorporates pathological and tumor biomarker data for individuals diagnosed with cancer or other conditions that are participants in well-defined epidemiological studies. A more detailed classification of tumors can be achieved by adding molecular annotation based on biomarker assessment in pathology specimens from patients in existing epidemiologic cohorts to existing clinical data available in these databases. On the other side, pathology studies are enriched by the principles of epidemiological methods to define study populations and design. Patho-epidemiology is uniquely derived from the interaction between investigators in these two disciplines.
1.2 Examples from the Intersection of Epidemiology and Pathology
One of the earliest cancer studies integrating the disciplines of epidemiology and pathology was undertaken to examine histological changes in lung tissue associated with exposure to passive smoking. This work was initiated following the publication of two seminal studies in 1983 showing nonsmoking women exposed to environmental tobacco from their husbands had an increased risk of lung cancer [3]. To provide follow-up evidence for this epidemiological finding, a team of epidemiologists and pathologists retrieved lung tissue at autopsy from a cohort of 283 adults not known to have died of cancer [4]. The team interviewed next of kin to collect data on the smoking habits of both the deceased individual and his/her family. A single pathologist reviewed the histological specimens without knowledge of the smoking status and characterized a variety of preneoplastic epithelial lesions. The study found nonsmoking women married to smoking husbands had a much higher prevalence of these precursor lesions in lung tissue than women married to nonsmoking husbands, and at a level similar to that of smoking women. The results from this study were pivotal in establishing the causal link between passive smoking and lung cancer risk.
1.2.1 Breast Cancer
Some of the clearest examples of the importance of integrating epidemiologists and pathologists working together to identify unique risk factors based on molecular subtypes are in breast cancer. Gene expression profiling studies identified unique molecular subtypes of breast cancer [5, 6], and these subtypes are not only prognostic and predictive, but also appear to be etiologically unique. Eliassen et al. [7] undertook a study to examine prediagnostic circulating levels of α-carotene, β-carotene, lycopene, and total carotenoids as related to breast cancer risk. Higher levels of each carotenoid were associated with a significant 18–28 % lower breast cancer risk during 20-year follow-up. Moreover, high plasma carotenoids were specifically associated with reduced risk for ER- cancers as well as cancers that were ultimately fatal.
1.2.2 Prostate Cancer
Prostate specific antigen (PSA), widely hailed as a victory in cancer screening, became a common screening test in the 1990s and eventually led to overdiagnosis and overtreatment of prostate cancer, causing some to advocate that routine screening be abandoned [8–10]. In the PSA screening era, detected prostate cancer is mainly indolent disease and there is a need for diagnostic measures that will narrow the focus on cancers with lethal potential, requiring that better surrogates be identified and validated [11]. Approaching this issue from the field of epidemiology, it appears that the risk factor patterns for potentially lethal prostate cancer differ greatly from that of indolent disease, suggesting different etiologies and distinct subtypes [12]. It was only through the integration of large epidemiologic databases and careful molecular annotation in prostate cancer specimens from these cohorts that novel molecular biomarkers such as gene expression profile signatures have been identified. For example, researchers identified a molecular signature of Gleason grade that strongly predicts lethal disease [13]. Gene expression profile signatures can also provide novel predictive tools to clinicians to decide on important therapeutic options.
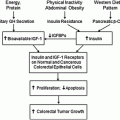
The somatic gene fusion TMPRSS2:ERG, involving the androgen-regulated TMPRSS2 and ERG (a member of the ETS family of oncogenes), has been proposed as a common prostate cancer molecular subtype that can readily be identified with molecular pathology techniques. A polymorphic CAG repeat sequence in the androgen receptor gene influences activity of the gene product. Men with shorter CAG repeats have a significantly higher risk of TMPRSS2:ERG prostate cancer, while there is no association of repeat length with TMPRSS2:ERG negative cancer [14]. There also exists a link between obesity and TMPRSS2:ERG, which stands out given the association of obesity and insulin signaling with prostate cancer mortality. TMPRSS2:ERG tumors have increased insulin and IGF1 expression. Likewise, obesity was strongly associated with mortality among patients whose tumors harbored TMPRSS2:ERG while no association was shown for men whose tumors lacked the gene fusion [15].
1.2.3 Glioblastoma
Great progress has been made in the field of glioblastoma (GBM), a cancer associated with very poor survival and high resistance to radiotherapy and chemotherapy. The identification of GBM patients also harboring IDH1 mutations, and the subsequent pathologic separation of these tumors from type I glioblastomas, has translated to effective stratification of prognostic groups and potential therapeutic opportunities. However, the identification of many risk factors associated with this disease (exposure to high doses of ionizing radiation, inherited mutations of highly penetrant genes associated with rare syndromes, etc.) have not yet translated to the identification of valuable therapeutic opportunities. Collaborative studies should continue to examine the interaction between exposures to therapeutic doses of ionizing radiation and identified signaling pathways disrupted in GBM (increased activation of receptor tyrosine kinase/RAS/PI3K signaling, loss of function in p53 signaling, and reduced signaling of the RB pathway) to identify links, potentially surrounding DNA repair genes [16].
1.2.4 Hematologic Malignancies
In the field of hematologic malignancies, epidemiologists and pathologists work together under the purview of the International Lymphoma Epidemiology Consortium (InterLymph) Pathology Working Group to facilitate uniformity in the investigation of lymphoma subtypes in epidemiologic research [17]. This initiative has completed significant work on non-genetic risk factors to determine end points for epidemiologic studies based on pathologic classification. InterLymph has also sought to delineate major risk factors associated with specific hematologic malignancies as well as across all hematologic malignancies. As an example, certain autoimmune diseases (e.g. systemic lupus erythematosus) are significantly associated with many hematologic malignancies [18]. However, there is a lack of data on risk factors for diffuse large B-cell lymphoma molecular subtypes, an area that would benefit from further collaborative work from this or other working groups.
The examples included thus far only scratch the surface of their respective fields. In order to provide a deep view of how the interactions between epidemiology and pathology can drive progress in a field, we will use colorectal cancer as a representative example.
1.2.5 Colorectal Cancer
Fearon and Vogelstein first described the classic model of colorectal carcinogenesis in 1990. Their model outlined the sequence of steps involved in the progression from normal epithelium, to benign precursors and into invasive adenocarcinoma [19]. Subsequently, additional genetic and epigenetic changes have been identified for colorectal carcinogenesis. Only some of these alterations are considered to be drivers of the development and progression of these cancers [20, 21]. Currently, colorectal cancer (CRC) has been characterized by three key molecular subtypes: chromosomal instability (CIN), microsatellite instability (MSI), and the CpG island methylator (CIMP) pathway [22–24]. CIN is the most common subtype, and is observed in approximately 80 % of the sporadic cases of CRC. The second major subtype, which accounts for approximately 10–15 % of CRC, is the CIMP subtype. Patho-epidemiology studies have helped to elucidate the effect of various exposures on CRC risk and survival.
An association between obesity and CRC risk overall is well established. In recent years, some studies have examined the association between measures of obesity and molecular subtypes or specific molecular alterations in CRC. Interestingly, based on studies conducted to date, obesity does not appear to act preferentially on many of the common molecular pathways identified for CRC. For example, obesity is not associated with risk of developing CRCs with high MSI that contains MLH1 methylation [25–28], BRAF mutation [27], CTNNB1 overexpression [29], or loss of expression in TP53 [30], in CDKN1B (or p27) [31], and in CDKN1A (or p21) [32]. Overall, it appears that obesity is not differentially related to tumors with MSI, but rather, may be even more strongly associated with those with a MS-stable phenotype. The timing of excess or deficiency of energy over the life course may be important. For example, in the Netherlands Cohort Study on Diet and Cancer, individuals who experienced severe war-related energy restriction during adolescence and early adulthood had a 35 % lower risk for CIMP-positive CRC [33]. It is unclear if severe energy restriction during the growth period would operate on similar mechanisms as excessive energy intake in adulthood.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree