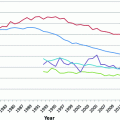
Fig. 29.1
Age-Specific Incidence of Hodgkin Lymphoma in the United States (SEER areas) by Sex and Race, 2007–2012. Source Howlader et al. [60] Rates for black males and black females aged 75 and older were not calculated due to small case numbers in those intervals
Worldwide, approximately 66,000 new diagnoses of HL are made each year, with an overall age-adjusted incidence rate of 0.9 per 100,000 [58]. Incidence is higher among more developed (2.1 per 100,000) versus less developed (0.6 per 100,000) regions, with the highest rates in Europe and the Americas, and the lowest rates in Africa, Asia and the Pacific (Fig. 29.2).
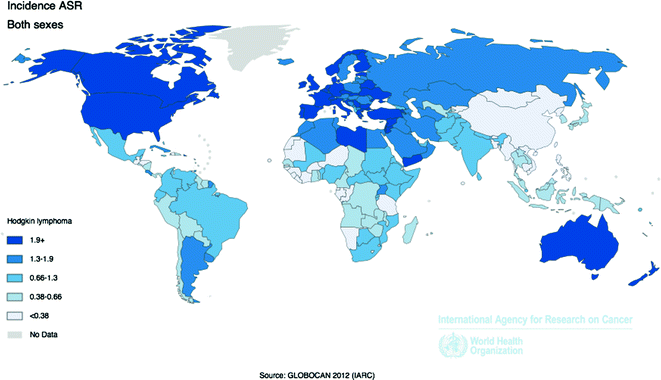

Fig. 29.2
Age-Standardized Incidence Rates for Hodgkin Lymphoma by Country for Men and Women Combined, 2012. Source World Health Organization, International Agency for Cancer Research, GLOBOCAN 2012
HL incidence by age displays a characteristic bimodal shape, with two peak incidence rates at young and older adulthood. In the U.S., HL is the eighth most commonly diagnosed cancer among children ages 0–14 years, and is the leading diagnosis among adolescents ages 15–19 [59]. According to Surveillance, Epidemiology, and End Results (SEER) estimates from 2007–2011, age specific incidence rates peak at 4.5 per 100,000 for age 20–24 years, fall to 2.4 per 100,000 for age 45–54 years, and increase again to 4.5–4.7 per 100,000 for age 70–84 years [60].
29.3.1.2 Non-Hodgkin Lymphoma
Overall, NHL is the sixth most commonly diagnosed cancer in the U.S. among both men and women. There were an estimated 71,850 new cases of NHL in the U.S. in 2015, accounting for more than 4 % of all cancer diagnoses (Fig. 29.3) [56, 60]. CLL is a leukemic condition that shares major pathological features with small lymphocytic lymphoma (SLL) and is classified with B-cell NHL. CLL accounted for an additional 14,620 NHL diagnoses in the U.S. in 2015 [60]. In addition, acute lymphoblastic leukemia (ALL) is a precursor lymphoid neoplasm of either pre-B (85 % of cases) or pre-T-cells (15 % of cases). In contrast to most other NHL subtypes, 75 % of ALL cases occur in children less than six years old, with more than 6,000 new cases diagnosed in the U.S. annually [26, 60]. The incidence rate for NHL is 19.7 per 100,000 men and women; however incidence rates for the various subtypes of NHL differ dramatically. Incidence rates of NHL in the U.S. and many other countries rose steadily during the second half of the twentieth century; the rate increases seem to have slowed overall in recent years, but the rising trend continues for the two most common subtypes, DLBCL and FL [61, 62].
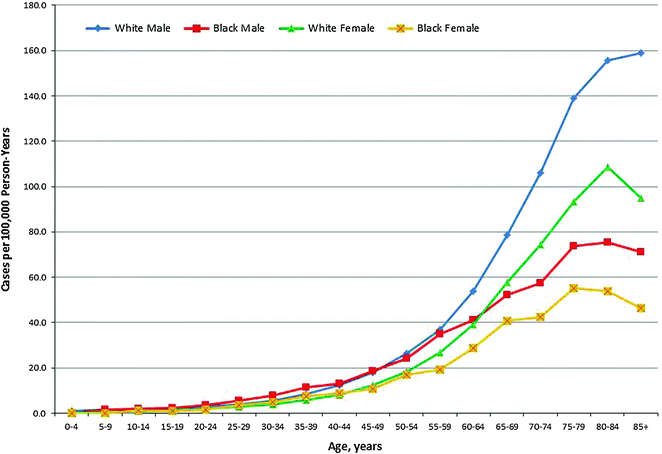

Fig. 29.3
Age-Specific Incidence of Non-Hodgkin Lymphoma in the United States (SEER areas) by Sex and Race, 2007–2012. Source Howlader et al. [60]
Worldwide, 385,741 cases of NHL were reported by IARC in 2012, with an age-standardized incidence rate of 5.0 per 100,000 people [58]. The more developed regions of the world experience a NHL incidence rate more than twice that of less developed regions, with the highest rates in the U.S., Western Europe, and Australia, and the lowest rates in Asia and Africa. However, the incidence of different NHL subtypes varies by geographic region; for example, DLBCL and FL are more common in North America and Europe than in Asia, whereas T-cell lymphomas are more common in Asia than in Western countries (Fig. 29.4) [61].
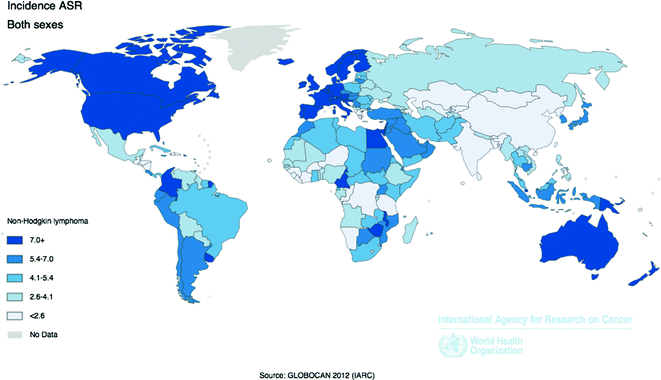

Fig. 29.4
Age-Standardized Incidence Rates for Non-Hodgkin Lymphoma by Country for Men and Women Combined, 2012. Source World Health Organization, International Agency for Cancer Research, GLOBOCAN 2012
29.3.1.3 Multiple Myeloma
An estimated 26,850 men and women in the U.S. were diagnosed with MM in 2015, accounting for more than 1.5 % of new cancer diagnoses, and more than 15 % of hematologic cancer diagnoses (Fig. 29.5) [60]. Based on data from the U.S. SEER Registry from 2008 to 2012, the overall incidence rate for MM is 6.3 cases per 100,000 men and women; however, men exhibit higher incidence rates than women (7.9 vs. 5.1 cases per 100,000), and African Americans have higher incidence rates (15.1 per 100,000 men and 11.2 per 100,000 women) compared to whites (7.5 per 100,000 men and 4.5 per 100,000 women) [60]. Studies suggest that people of African or African American descent also have twice the prevalence of precursor MGUS compared to white and Hispanic populations [46, 63, 64].
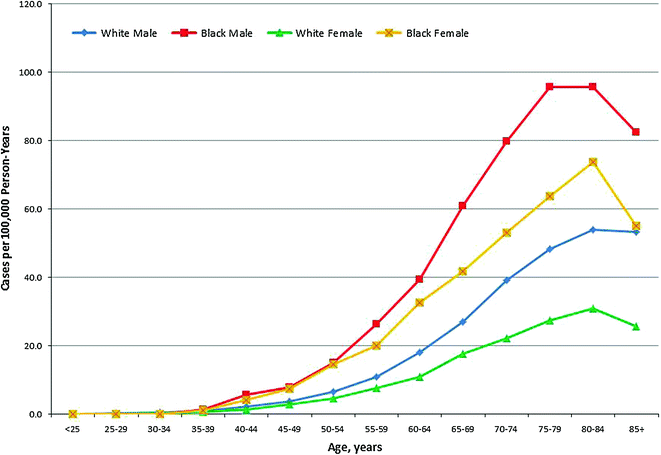

Fig. 29.5
Age-Specific Incidence of Multiple Myeloma in the United States (SEER areas) by Sex and Race, 2007–2012. Source Howlader et al. [60]
Most cases of MM are diagnosed in adults age 65–74 years, with a median age at diagnosis of 69 years [60]. Only about 3 % of cases are diagnosed in people younger than 40 years old [65, 66]. Older age at diagnosis is associated with poorer survival, as well as more advanced stage at diagnosis [67]. Conversely, younger patients often present at an earlier stage, and have less frequent adverse prognostic factors, such as low hemoglobin and high C-reactive protein [66]. As the population ages, more adults are expected to be diagnosed with MM, and the elderly will likely comprise a larger proportion of patients [68, 69].
29.3.2 Mortality
29.3.2.1 Hodgkin Lymphoma
An estimated 1150 HL deaths occurred in the U.S. in 2015, with death rates steadily declining over the past four decades [56]. The 5-year relative survival rate for patients diagnosed between 2004 and 2010 was 85 %, with a 94 % survival for patients diagnosed at less than 45 years of age versus 53 % for patients diagnosed at 65 years of age or older [57]. Five-year survival rates were generally similar between men (84 %) and women (86 %), and between Caucasian (86 %) and African American (82 %) patients. Worldwide, approximately 25,500 people die annually due to HL [58]. Age-standardized mortality rates are similar in more developed and less developed regions of the world (0.3 per 100,000).
Among long-term survivors of HL, second primary malignancies, largely due to complications of primary HL therapy, are a major cause of morbidity and mortality [70]. Among the highest relative risks are second hematologic malignancies, particularly leukemia and NHL [71, 72]. HL survivors also have an excess risk of certain solid cancers compared to the general population, including breast and colorectal cancers [73].
29.3.2.2 Non-Hodgkin Lymphoma
NHL is the ninth most common cause of cancer death in men, and the eighth most common cause of cancer death in women in the U.S., with approximately 20,000 deaths expected in 2015, accounting for 3–4 % of all cancer deaths [56, 60].
About 200,000 people worldwide die from NHL annually, with an age-standardized mortality rate of 2.5 per 100,000 men and women. Overall, mortality rates are somewhat more uniform between countries, in contrast to incidence rates, although slightly higher mortality rates are observed in the Middle East and Africa, and lower mortality rates are observed in Asia and Europe [58]. For all NHL subtypes combined, 70 % of people will survive at least 5 years following diagnosis [60]. However, as observed for incidence rates, mortality rates vary considerably across histologic types and tumor molecular subtypes [22].
29.3.2.3 Multiple Myeloma
Approximately 11,000 deaths due to MM occur in the U.S. annually, accounting for more than 2 % of cancer deaths, with a median age at death from MM of 75 years [60]. Following decades of unchanged survival rates, the development of new MM treatments in the early 2000s, including immunomodulatory drugs and protease inhibitors, has led to marked increases in survival among MM patients of all age groups [74–77]. Between 1995 and 2001, 5-year relative survival rates for MM were only 32 %, with slightly higher rates for men [60]. According to more recent analyses from the U.S. SEER Registry, 47 % (2005–2011) to 53 % (2008–2010) of patients diagnosed with MM now survive at least 5 years, an increase of more than 14 % over a ten-year period, although disparities between ethnic groups persist [60, 78, 79]. A study of 1038 patients from the Mayo Clinic observed improved survival for patients diagnosed between 2006 and 2010 compared to those diagnosed just a few years earlier (2001–2005), with the largest improvements among patients aged older than 65 years [80].
Worldwide, over 114,250 incident cases of MM were diagnosed in 2012, and over 80,000 people died from the disease, accounting for less than 1 % of all new cancer cases and cancer deaths (Fig. 29.6). The highest incidence and mortality rates occur in more developed countries compared to less developed countries [58]. The U.S. and Europe have similar MM incidence (3.6 and 2.6 per 100,000, respectively) and mortality (1.9 and 1.4 per 100,000) rates, more than twice the rates observed in Africa and Asia [58]. Some of the geographical differences in incidence and mortality rates may be due to detection bias, resulting from the challenges of diagnosing MM in low resource settings [81].
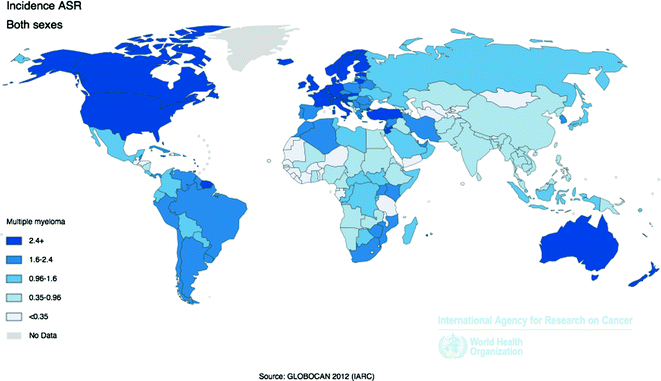

Fig. 29.6
Age-Standardized Incidence Rates for Multiple Myeloma by Country for Men and Women Combined, 2012. Source World Health Organization, International Agency for Cancer Research, GLOBOCAN 2012
29.4 Risk Factors for Hematologic Malignancies
29.4.1 Risk Factors for Hodgkin Lymphoma
29.4.1.1 Introduction
The most consistent risk factors for HL are family history of HL and delayed exposure to the Epstein-Barr virus (EBV). More modest associations with increased risk of HL have been reported for childhood social environment, smoking, and higher BMI, whereas aspirin has been associated with reduced risk of HL. Each of these HL risk factors is hypothesized to act via immune-mediating mechanisms, such as by influencing the inflammatory milieu or by delaying the maturation of the immune responses that protect against the oncogenic effects of infectious agents like EBV. The following section will focus on risk factors for classical HL since NLPHL has a different pathogenesis and natural history [14].
29.4.1.2 Family History and Genetic Susceptibility
Risk of HL in monozygotic twins with one twin affected is approximately 100-fold higher than the general population [82]. A more modest 4-fold increased risk of HL is seen among first-degree relatives of HL patients [83]. Furthermore, risk of HL is higher among relatives with an affected sibling compared to an affected parent [84]. Family history may increase risk of HL through shared childhood exposures, such as EBV infection, common inherited genetic susceptibility, or a combination of these factors.
Evidence from family as well as population-based studies has indicated a consistent association between human leukocyte antigen (HLA) type and HL risk [85]. Genome-wide association studies (GWAS) have replicated an association between genetic variation in HLA-related genes and HL risk, while also identifying a limited number of novel susceptibility loci [86–88].
29.4.1.3 Infections
As noted above, exposure (and timing of exposure) to common childhood infections may influence risk of HL [89–94]. Hypotheses focus in particular on EBV infection, a ubiquitous herpesvirus and well-studied infectious risk factor for HL. Delayed first exposure to EBV can lead to infectious mononucleosis, which is characterized by EBV antibody profiles that indicate poor host immune control of EBV, similar to persons with severe or chronic EBV [95, 96]. It is plausible that such persons have diminished immune protection against the known oncogenic effects of EBV [97, 98]. Several cohort studies performed in the 1970s indicated a consistent 3-fold increased risk of HL in young adults with serologically confirmed infectious mononucleosis [99]. Subsequent studies have also shown an association between history of infectious mononucleosis and HL risk [100], as well as an approximately 3 to 4-fold higher risk for developing EBV-positive HL [101, 102]. Of interest, one serologic study suggested that chronic or more severe EBV is a risk factor for HL risk independent of history of infectious mononucleosis [97], confirming other studies of EBV serology and HL risk [98]. Furthermore, the EBV genome and expression of EBV-encoded latent gene products have been detected in approximately one third to more than two thirds of HL tumors depending on histologic subtype [103–108]. Whether EBV contributes to the etiology or pathogenesis of EBV-negative HL cases is unclear.
Human immunodeficiency virus (HIV)-infected individuals are at increased risk of developing HL as well as certain subtypes of NHL, including DLBCL and Burkitt lymphoma [109, 110]. Numerous other viral infections have been explored in relation to HL risk, though no consistent associations have been reported [111–113].
29.4.1.4 Lifestyle Factors
Childhood environment. Many studies have shown a link between HL risk and factors related to childhood environment, which are hypothesized to reflect the timing of exposure to common infectious agents. The timing of such exposures is believed to influence underlying immune competency, especially with regard to the maturation of the immune responses that protect against the oncogenic effect of infections; those immune responses are not well developed at birth but are understood to be induced and matured through exposure to common infections during childhood [114, 115]. Several studies have linked increased sibship size and later birth order, each of which would increase the chances for earlier exposure to common infections, with reduced risk of HL in young adulthood [89, 91–94]. Nursery school or daycare attendance for at least one year, which could also increase the likelihood of (earlier) exposure to infections, was also associated with a reduced risk of HL among young adults in a large case-control study in Massachusetts and Connecticut [90]. Taken together, these studies suggest that decreased or delayed exposure to common childhood infection may increase risk of HL, possibly due to their underlying influence on host immune system maturation.
Aspirin. Regular use of aspirin, which has anti-inflammatory properties, was inversely associated with risk of HL in a large case-control study, whereas acetaminophen use was positively associated with HL risk [116]. A nationwide study of more than 6 million Danish residents also reported a modest inverse association between long-term aspirin use and HL risk, and a positive association for selective cyclooxygenase-2 inhibitors and other non-steroidal anti-inflammatory drugs with HL risk [117].
Smoking. A number of studies have linked smoking with increased risk of HL [90, 118–121]. In addition, a pooled analysis conducted by the InterLymph Consortium, including 3335 HL cases, reported a modest 10 % increased risk of HL among ever versus never smokers, particularly for mixed cellularity HL (Odds Ratio [OR] 1.60, 95 % Confidence Interval [CI] 1.29–1.99) and EBV-positive HL (OR 1.81, 95 % CI 1.27–2.56) among current smokers [122].
Obesity. Studies of body mass index (BMI) and HL have shown mixed results. In a meta-analysis of 7 prospective studies, the association with HL risk was null for BMI values considered to indicate overweight, but positive for BMI values associated with obesity (summary relative risk [RR] 1.41, 95 % CI 1.14–1.75) [123], a condition characterized by heightened inflammation and deregulation of several immune mediators and growth factors. A large cohort study of 1.3 million women in the United Kingdom, including 267 HL cases, reported that higher BMI was associated with an increased risk of HL [124]. In a case-control study, BMI was positively associated with HL among women aged 19–44 years, but inversely associated with HL risk at older ages [125]. In another case-control study, increasing BMI was associated with higher risk of HL among women younger than 35 years, but lower risk of HL among women aged 35 years or older; no association was found among men [126].
Diet. Associations between dietary factors and HL risk have been reported in few studies. A large case-control study of dietary patterns found that a diet high in desserts/sweets was associated with younger adult and EBV-negative, younger adult HL risk, while a diet high in meat was associated with older adult and EBV-negative, older adult HL risk [127]. The same case-control study reported a positive association of dietary intake of saturated fat, and an inverse association of monounsaturated fat, with younger adult HL risk [128]. A “Western” style dietary pattern and diets high in saturated fat have been associated with pro-inflammatory factors [129, 130], thereby suggesting a potential immune-modulating mechanism of diet on HL risk. Alcohol intake has been associated with reduced risk of HL in four studies [121, 131–133], while a fifth study found no association [120]. Potential mechanisms for alcohol and reduced risk of HL include improved immune function and the antioxidant properties of certain alcoholic beverages [134]. Additional dietary studies of HL risk have shown largely null or modest associations [135–139].
29.4.1.5 Occupational and Environmental Exposures
Very little evidence exists linking occupational or environmental chemical exposures to HL risk. Weak positive associations have been noted for wood-related exposures and woodworking occupations, though many studies found no association [reviewed in 15]. Studies involving workplace exposure to chemicals have also been inconclusive [reviewed in 140].
29.4.2 Risk Factors for Non-Hodgkin Lymphoma
29.4.2.1 Introduction
The most established risk factor for NHL is severe immune compromise, such as that associated with HIV infection or post-transplant medication use. However, most cases of NHL occur in persons with no overt immune compromise, suggesting that other immune-modulating exposures influence their development. NHL is a group of etiologically and epidemiologically distinct disease subtypes; as such, reported associations between some risk factors and NHL appear to vary by subtype. Other risk factors appear to be consistently associated with risk of most NHL subtypes, including family history of hematologic cancer, autoimmune and atopic diseases, certain lifestyle factors, and sun exposure [141–143]. The incidence of most NHL subtypes increases with age; however, certain subtypes, such as endemic Burkitt lymphoma and ALL, are more prevalent among children and adolescents. Overall, men experience higher incidence rates of NHL compared to women, but some histologic types do not demonstrate gender differences in incidence rates. The following section will summarize the literature on NHL risk factors, focusing on associations with the most common subtypes of the disease to reflect the significant etiologic complexity of NHL [141].
29.4.2.2 Family History and Genetic Susceptibility
Individuals with a family history of a first-degree relative diagnosed with HL and NHL, leukemia, or MM are at an increased risk for being diagnosed with NHL; in particular, a family history of NHL or HL is associated with an increased risk of a B-cell or T-cell NHL diagnosis [141]. A family history of MM is associated with an increased risk of mycosis fungoides and Sézary syndrome, which are T-cell lymphomas that originate in the skin [24]. Individual studies of candidate genes and also pooled genetic studies by the InterLymph Consortium have reported genetic susceptibility from common variants in genes related to the host immune response, cell cycle control, DNA repair and other plausible pathways [144–150]; more recently, numerous novel susceptibility loci in immunoregulatory, human leukocyte antigen-related, and other genes have been reported for several major histologic types of NHL by the ongoing, large international GWAS of NHL [151–154].
29.4.2.3 Immunosuppression, Autoimmune Disease, and Infections
Immunosuppression has long been associated with an increased risk of NHL. NHL is among the most common cancers diagnosed in recipients of solid organ transplant and is an AIDS-defining malignancy in individuals infected with HIV [155–158]. Furthermore, individuals diagnosed with certain autoimmune diseases, including rheumatoid arthritis, systemic lupus erythematosus, and Sjögren’s syndrome, are at an increased risk for NHL [159, 160]. Allergies, hay fever, or atopic disease have been associated with a reduced risk of several NHL subtypes including FL, CLL/SLL, peripheral T-cell lymphomas, mantle cell lymphoma, LPL/WM and sporadic Burkitt lymphoma (i.e. non-African Burkitt lymphoma) [161–166]. In contrast, eczema may increase the risk of T-cell lymphomas and sporadic Burkitt lymphoma. Given that some of the evidence for the allergy/atopy association with NHL derives from case-control studies, prospective data are needed to assess the influence of subclinical disease (i.e., reverse causality) on the published observations.
Several subtypes are associated with infection by a specific agent. For example, human T-cell lymphotropic virus-type 1 (HTLV-1) is associated with and a necessary cause of ATL, although incidence varies greatly by geographic area among HTLV-1-endemic populations [167, 168]. Also, infection with Helicobacter pylori is associated with an increased risk of MALT lymphoma [169], and infection with EBV is associated with 97 % of endemic Burkitt lymphoma tumors in equatorial Africa [170, 171]. Sporadic Burkitt lymphoma does not exhibit such a strong causal relationship with EBV, and is often diagnosed at older ages [172, 173]. EBV has also been associated with other subtypes of NHL, although generally not with most NHL [174]. Infection with Hepatitis C (HCV) has also been associated with an increased risk of B-cell NHL, including DLBCL, CLL/SLL, LPL/WM, marginal zone lymphoma and sporadic Burkitt lymphoma in populations with higher HCV prevalence [141, 163, 165, 166, 175–177].
29.4.2.4 Lifestyle Factors
Obesity/Physical Activity. Several studies have reported an association between obesity and other anthropometric factors and NHL risk [178]; however, published associations across specific subtypes are inconsistent, and thus reported associations with all NHL types combined are challenging to interpret. No association was reported between obesity and all NHL types combined in a pooled analysis of 18 case-control studies; however, an 80 % increased risk of DLBCL was associated with severe obesity (BMI ≥ 40 kg/m2) [179]. A meta-analysis of prospective studies reported a 7 % increased risk of all NHL combined, and a 14 % increased risk of overall NHL mortality associated with a 5 kg/m2 increase in BMI. When restricted to studies reporting NHL subtype, increased BMI was only significantly associated with an increased risk of DLBCL [123]. Higher young adult BMI has also been associated with an increased risk of DLBCL and FL [161, 177]. In summary, a relationship between obesity and NHL risk appears strongest among patients diagnosed with DLBCL, the most commonly diagnosed NHL subtype; it is less clear whether obesity is associated with risk of (or mortality from) other histologic types.
Studies have not consistently reported an association between physical activity and NHL risk, overall or by subtype [180, 181]. A recent meta-analysis of 23 studies observed a borderline significant 9 % decreased risk of all NHL subtypes combined when comparing high versus low physical activity levels, but found no association with risk of DLBCL or FL subtype [182].
Sun Exposure and Vitamin D. Several epidemiologic studies have reported a positive association between high levels of ambient ultraviolet (UV) exposure and NHL risk, with variation by subtype and race/ethnicity [183]; however, other studies have not observed such an association. In contrast, a large international pooled study by the InterLymph Consortium recently reported an inverse association of recreational sun exposure with risk of NHL that did not demonstrate significant variability by histologic type [141]. A strong relationship between dietary or serum Vitamin D and NHL risk is not apparent in the literature [142, 143, 184, 185].
Diet. Analyses of diet and NHL risk have been inconsistent and vary by subtype. Studies of dietary pattern suggest a positive association between a Western-style diet high in fats and meat and risk of NHL, in particular an increased risk of FL [186, 187], while a Mediterranean diet was not associated with NHL risk [188]. These findings are consistent with other reports of an increased risk of NHL in persons with higher consumption of total and trans fats, and of red meats [189]. Dietary phytocompounds were not associated with B-cell NHL in a cohort of women [135]. Relatively consistent reports suggest an inverse association of vegetables [190, 191] and of marine fatty acids [192] and risk of some NHL subtypes.
Smoking. Smoking is associated with some NHL subtypes, and within those subtypes, has shown variation by anatomical site. For example, smoking is associated with DLBCL that presents in the CNS [193]. Cigarette smoking is associated with an increased risk of FL, mycosis fungoides and Sézary syndrome [162, 165], peripheral T-cell lymphoma, and LPL/WM, and an inverse association with the rare subtype hairy cell leukemia [24, 141, 161, 194].
Alcohol. A pooled analysis of 9 international case-control studies reported a protective association between people who currently drink alcohol and risk of NHL, compared to non-drinkers, with weaker associations for former drinkers [195]. The authors did not observe a dose-response relationship between the amount of alcohol consumed and NHL risk. An updated pooled analysis of case-control studies reported protective associations between ever-drinkers of alcohol and risk of overall NHL, DLBCL, and FL, when compared to nondrinkers, although no association was observed for risk of MZL or CLL/SLL [141]. However, a prospective analysis found an elevated risk of overall B-cell NHL and of FL in women who were former alcohol drinkers, suggesting the timing of alcohol consumption, as well as factors associated with quitting drinking, may be relevant to lymphomagenesis [196].
29.4.2.5 Occupational and Environmental Exposures
A large European study including 866 cases of NHL observed an increased risk of disease among car repair workers and butchers, possibly reflecting exposure to solvents or zoonotic viruses [197]. A pooled analysis of four case-control studies including more than 3700 cases of NHL observed an increased risk associated with occupational exposure to trichloroethylene (TCE) [198]. Similarly, a meta-analysis of cohort and case-control studies also observed an increased risk of NHL with occupational TCE exposure [199].
Teachers may have a reduced risk of being diagnosed with multiple subtypes of NHL, including marginal zone and FL, while painters and farm workers may be at an increased risk [141, 200]. Positive associations have been observed between farm workers and DLBCL particularly among women, CLL/SLL, hairy cell leukemia, mantle cell lymphoma, and mycosis fungoides and Sézary syndrome. An inverse association was observed between farm work and peripheral T-cell lymphoma in a pooled analysis of 15 case-control studies [24, 162, 164, 166, 177, 194]. Other occupations with consistent positive associations reported with NHL subtypes, including DLBCL, across studies include seamstress or embroiderer and hairdresser [162, 166, 177]. Reported associations between NHL risk and exposure to lead or cadmium have been inconsistent [201, 202].
29.4.3 Risk Factors for Multiple Myeloma
29.4.3.1 Introduction
Few modifiable risk factors for MM have been identified. Since this malignancy is often diagnosed at an advanced stage, and survival rates remain modest despite recent improvements in treatment, identification of risk factors and means of prevention are paramount to reducing the burden of MM. In addition to the growing literature linking genetic markers to MM risk and survival, characterization of the role of cytokine and growth factor signaling in the tumor microenvironment is becoming increasingly important to understanding myeloma pathogenesis. In particular, insights from the well-characterized pathogenic roles for some cytokine and growth factor pathways may inform etiologic hypotheses. The following section summarizes the epidemiologic evidence elucidating risk factors for MM.
29.4.3.2 Family History and Genetic Susceptibility
Individuals with a first-degree relative diagnosed with MGUS or MM have a 2–3 fold increased risk of developing either disease themselves [48, 203]. A family history of B-cell lymphoproliferative diseases is also associated with an increased risk of MM [204, 205]. Studies of familial MM have also observed an increased incidence of both hematologic and solid tumors in relatives of patients with MM, as well as an early age at myeloma diagnosis in successive generations (i.e. “anticipation”), further suggesting a role for genetic heritability [206].
Two GWAS identified 7 single nucleotide polymorphisms (SNP) significantly associated with MM risk — 6 SNPs were positively associated and 1 SNP was inversely associated with myeloma risk [207–210]. The first GWAS of MM survival identified variants at 16p13 near the gene FOPNL associated with survival [211]. Certain genetic mutations and chromosomal aberrations have been consistently associated with poor survival among patients with MM, including t(4;14) and 17p13 deletions [75, 212, 213]. Evidence is inconclusive for t(14;16) and chromosome 1q21 amplification [75].
Variability in several genes has been associated with an increased risk of MM; however, the 11 strongest associations reported between specific SNPs and MM risk from candidate gene studies were not validated in a recent analysis by the International Multiple Myeloma rESEarch (IMMEnSE) consortium [214]. Variability in genes that encode molecules on the insulin-like growth factor (IGF)-1 and interleukin (IL)-6 signaling pathways, including the gene encoding insulin receptor substrate-1 (IRS-1), and the gene encoding IL-6 (IL6) and the IL-6 receptor (IL6R), have been associated with risk of MM in U.S. studies [215, 216] supporting earlier investigations linking immune system modulation and serum levels of cytokines to MM development [217, 218]. Numerous prognostic gene expression profiling signatures for MM have been reported; currently, the IMWG is working to unify the prognostic signatures through prognostic modeling [75].
Cytokine and growth factor signaling in the tumor microenvironment may also be important to myeloma development, and this is a developing area of interest for etiologic study. Prediagnostic serologic levels of IGF-binding protein (BP)-1 were positively associated with MM risk within 3 years of diagnosis, and sIL-6R with MM risk within 6 years of diagnosis in a pooled analysis of 8 cohort studies [219].
29.4.3.3 Lifestyle Factors
Obesity/Physical Activity. When considering the associations between potentially modifiable lifestyle factors and MM, the most consistently reported association has been that between obesity and MM. A pooled analysis of 20 prospective studies including 1388 MM deaths observed a positive association of mortality from myeloma with both young-adult and cohort-entry BMI. A separate positive association was observed between MM mortality and high waist circumference. Women who reported both young-adult BMI > 25 and cohort-entry BMI > 30 had a nearly 2-fold increased risk of dying from MM compared to women with normal BMI at both time points; this association was not evident in men [220]. Obesity and overweight BMI have also been associated with an increased risk of incident myeloma [181, 221]. Of note, these findings for BMI are consistent with the aforementioned data from serologic studies of other hormonal pathways that are altered in obese individuals and important in MM pathogenesis. Data on physical activity and sedentary behavior is more limited, but suggest an inverse association with physical activity and a positive association with increased sitting time [181, 221, 222].
The findings for energy balance-related risk factors appear to be corroborated by biomarker studies, including the observation that circulating adipokines, which are cytokines secreted by adipose tissue, may play a role in myelomagenesis, and that adiponectin levels in particular are inversely associated with myeloma risk in several studies [223–225].
Aspirin. Aspirin use has been associated with a significant 37–39 % reduced risk of MM in a prospective analysis, among adults taking ≥5 adult strength tablets per week, and those with ≥11 years of continuous use [226]. Although another large prospective study did not observe an association of aspirin use with MM risk [227], the aforementioned findings are plausible given the known role of NF-κB-mediated pathways in MM pathogenesis and the down-regulatory effect of aspirin on NF-κB and several of its downstream targets [228–230]. However, a hospital-based case-control study did not observe an association between aspirin use and MM risk, although regular acetaminophen use was associated with a nearly 3-fold increased risk of myeloma in that population [231].
Alcohol and Smoking. A pooled analysis of 6 case-control studies including 1567 MM cases suggests an inverse association between self-reported alcohol use and myeloma risk [232]. A separate pooled analysis of 9 international case-control studies including 2670 cases found no evidence of an association between cigarette smoking and risk of MM [233].
29.4.3.4 Occupational and Environmental Exposures
Reports of positive associations between MM risk and a number of environmental and occupational exposures have long been suggested in the literature. Studies have focused on agricultural workers, employees of the petroleum and leather industries, cosmetologists, and firefighters, with the most consistent associations reported following exposure to pesticides [234–242]. Studies have also reported a suggested increased risk of MM in radiation workers [243–245] and among hairdressers and those exposed to hair dye [246]. However, the associations between occupational and environmental factors and MM risk have been modest and inconsistent across studies, possibly due to inconsistent or biased methods for assessing exposure, inconsistent ranges of exposure across populations, or perhaps suggesting these factors may not be strongly related to MM.
29.5 Summary
Together, hematologic malignancies account for about 10 % of all cancers diagnosed in the U.S. each year [56]. However, hematologic malignancies are in fact a diverse group of diseases, with distinct combinations of cell of origin, clinical presentation, descriptive epidemiology, and known risk factors, as detailed above. The emerging knowledge of molecular subtypes within specific histologic types of these tumors further adds complexity to the study of their etiology and prevention. Several of these cancers share common aspects in their pathogenesis, for example a role for inflammation and immune activation, even if specific signaling pathway alterations may vary across types. As a result, a given immune-modulating risk factor, such as autoimmune disease, infection, obesity or immunosuppression may demonstrate an association with more than one type, but not necessarily with all types, of hematologic cancer.
As a group, hematologic malignancies appear to share some epidemiologic risk factors, such as a family history of hematologic cancer. The knowledge of increased risk among first-degree relatives of hematologic cancer or MGUS patients further underscores the urgency of identifying modifiable risk factors and prevention strategies for these malignancies. However, to date many putative risk factors do not show a universal association with all subtypes. For example, while certain histologic subtypes of both HL and NHL have been linked to infection with EBV, others show no association with infectious agents. Some tumors have known and increasingly well-characterized pre-malignant conditions that are believed to precede all malignant diagnoses (i.e., MGUS prior to MM or WM), but we know very little regarding risk factors for progression from pre-malignancy. Similarly, we do not know whether putative risk factors vary between more and less aggressive molecular subtypes that have been identified and found to hold clinical relevance for some histologic types of hematologic cancer (such as DLBCL, CLL, FL and others). Studies that incorporate the collection and molecular interrogation of diagnostic tumor tissue, and that evaluate risk factors separately by molecular type, will likely offer valuable insights regarding opportunities to prevent and diminish the severity of those tumors. Further, much of the current understanding of risk factors for hematologic malignancies derives from case-control studies that are vulnerable to survival, recall and other biases such as reverse causality. Thus, clarification of purported etiologic associations and exploration of questions such as the most relevant timing of exposure for influencing disease risk are urgently needed from prospective cohort studies.
References
1.
Ma X. Epidemiology of myelodysplastic syndromes. Am J Med. 2012;125(7 Suppl):S2–5.PubMedPubMedCentral
3.
Dohner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136–52.PubMed
4.
Rubnitz JE, Gibson B, Smith FO. Acute myeloid leukemia. Pediatr Clin N Am. 2008;55(1):21–51, ix.
5.
Hoglund M, Sandin F, Simonsson B. Epidemiology of chronic myeloid leukaemia: an update. Ann Hematol. 2015;94(Suppl 2):S241–7.PubMed
6.
Linet MS, Devesa SS, Morgan GJ. The Leukemias. In: Schottenfeld D, Fraumeni Jr. JF, editors. Cancer Epidemiology and Prevention, 3rd ed. New York. Oxford University Press; 2006.
7.
Visser O, Trama A, Maynadie M, Stiller C, Marcos-Gragera R, De Angelis R, et al. Incidence, survival and prevalence of myeloid malignancies in Europe. Eur J Cancer. 2012;48(17):3257–66.PubMed
8.
Jaffe ES, Harris NL, Stein H, Vardiman JW (eds). Pathology and genetics of tumours of haematopoietic and lymphoid tissues. World Health Organization Classification of Tumours. Lyon, France: IARC Press 2001.
9.
Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon, France: IARC Press; 2008.
10.
Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision to the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–90.
11.
Morton LM, Turner JJ, Cerhan JR, Linet MS, Treseler PA, Clarke CA, et al. Proposed classification of lymphoid neoplasms for epidemiologic research from the pathology working group of the international lymphoma epidemiology consortium (InterLymph). Blood. 2007;110(2):695–708.PubMedPubMedCentral
12.
Turner JJ, Morton LM, Linet MS, Clarke CA, Kadin ME, Vajdic CM, et al. InterLymph hierarchical classification of lymphoid neoplasms for epidemiologic research based on the WHO classification (2008): update and future directions. Blood. 2010;116(20):e90–8.PubMedPubMedCentral
13.
Gobbi PG, Ferreri AJ, Ponzoni M, Levis A. Hodgkin lymphoma. Crit Rev Oncol Hematol. 2013;85(2):216–37.PubMed
14.
Hoppe RT, Advani RH, Ai WZ, Ambinder RF, Aoun P, Bello CM, et al. Hodgkin lymphoma, version 2.2015. J Nat Compr Cancer Netw JNCCN. 2015;13(5):554–86.
15.
Mueller NE, Grufferman S. Hodgkin lymphoma. In: Schottenfeld D, Fraumeni Jr. JF, editors. Cancer epidemiology and prevention, 3rd ed. New York. Oxford University Press;2006.
16.
Marshall NA, Christie LE, Munro LR, Culligan DJ, Johnston PW, Barker RN, et al. Immunosuppressive regulatory T cells are abundant in the reactive lymphocytes of Hodgkin lymphoma. Blood. 2004;103(5):1755–62.PubMed