Fig. 11.1
Age incidence and mortality curves for breast cancer in the U.S. for African American women and non-Hispanic white women (SEER Research Data 1973–2012; Fast Stats: an interactive tool for access to SEER cancer statistics. Surveillance Research Program, National Cancer Institute. http://seer.cancer.gov/faststats.) (Accessed on 11-21-2015)
Breast cancer is the second leading cause of cancer death among U.S. women, after lung cancer, which is the leading cause of cancer death among women [2]. Approximately 40,000 women die per year from breast cancer [4]. Breast cancer is the leading cause of death among women age 40–55 [2]. However, the lifetime risk of death from breast cancer is low (3.4%) [5]. As of 2012, there are approximately 2.9 million breast cancer survivors (i.e., women who have been diagnosed with breast cancer who are alive) in the U.S. [4].
Breast cancer incidence and mortality rates vary significantly by race and ethnicity. Hispanic, Asian, and American Indian women have the lowest incidence rates of breast cancer, while white and African American women have the highest incidence rates. Overall, white U.S. women have the highest lifetime risk (12.8 %) of breast cancer, while African American women have a slightly lower life time risk of 10.1 % [6]. However, African American women have higher incidence rates before age 40 and also have higher rates of more aggressive breast cancer subtypes such as estrogen receptor negative (ER−) breast cancers compared with white women [7]. White women also have the highest age-adjusted incidence rate (137 per 100,000 vs. 118 per 100,000) compared to all other race/ethnicities. Although white women have higher lifetime risk and age-adjusted incidence rates, they have lower mortality rates than African American women (Fig. 11.2) [6, 7]. African American women have a 3.4 % (30.8 per 100,000) risk of dying from breast cancer compared to a 3.1 % (22.7 per 100,000) for white women [5, 6].
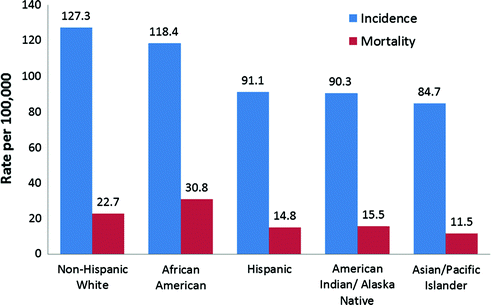

Fig. 11.2
Breast Cancer Incidence and Mortality Rates (per 100,000) by Race and Ethnicity (SEER Research Data 1973-2012; Fast Stats: An interactive tool for access to SEER cancer statistics. Surveillance Research Program, National Cancer Institute. http://seer.cancer.gov/faststats.) (Accessed on 11-21-2015)
11.2.2 Incidence and Mortality Trends
Breast cancer incidence (Fig. 11.3) and mortality trends (Fig. 11.4) have varied over time in the U.S. Breast cancer incidence has increased in all age groups since 1930, averaging 1.4 % increase in age-adjusted incidence per year from 1950–2000 [8]. This increase has increased more sharply in older women and young African American women (22 %) [6, 7]. Increases since the 1930s may be attributable to the changing prevalence of breast cancer risk factors including changes in reproductive patterns, increasing exogenous hormone use, and increasing postmenopausal body mass index (BMI). Moreover, increases in incidence rates seen since the 1980s can be attributed in part to widespread uptake of screening mammography [9–14]. Additionally, from 2001 to 2004, a decrease in incidence rates (3.5 %) occurred possibly due to the 2002 Women’s Health Initiative (WHI) finding that estrogen plus progestin menopausal hormone therapy increased breast cancer risk for postmenopausal women (See Sect. 11.4.6) [8, 15]. From 2006 to 2010, overall breast cancer incidence rates increased slightly among African Americans (0.2 % per year) and decreased among Hispanic women, with no change in other racial groups [7].
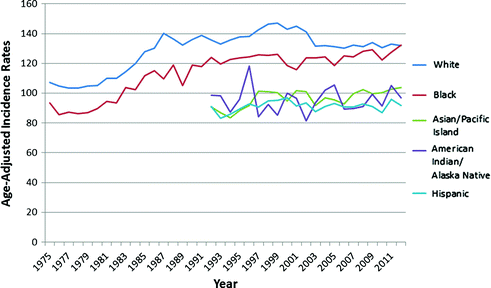
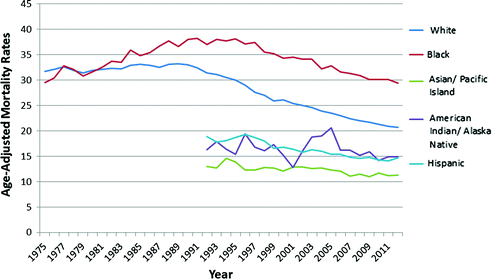

Fig. 11.3
Age-Adjusted Incidence Rates (per 100,000) of Female Breast Cancer from 1975–2012 (SEER Research Data 1973-2012; Fast Stats: An interactive tool for access to SEER cancer statistics. Surveillance Research Program, National Cancer Institute. http://seer.cancer.gov/faststats.) (Accessed on 11-21-2015)

Fig. 11.4
Age-Adjusted Mortality Rates (per 100,000) of Female Breast Cancer from 1975–2012 (SEER Research Data 1973-2012; Fast Stats: An interactive tool for access to SEER cancer statistics. Surveillance Research Program, National Cancer Institute. http://seer.cancer.gov/faststats.) (Accessed on 11-21-2015)
In 2008, breast cancer incidence rates varied by more than 13-fold [16], with the highest rates observed in Europe and North America and the lowest rates in Asia. This likely reflects a combination of differences in breast cancer screening, reporting, and risk factor exposure rates. Migrant studies, in which changes in breast cancer rates are evaluated in women who move from low- to high-risk countries—or vice versa—have shown that the rates of the host country are assumed over time, frequently one or two generations later [17, 18]. These data indicate that international differences in breast cancer rates may be due, at least in part, to environmental and lifestyle differences.
Age-adjusted mortality rates were stable from 1950s to 1980s [19], with a slight decrease in mortality in the 1980s–1990s likely due to advances in treatment and screening [3]. Mortality rates from the 1970s to 1990s continued to decrease for young and old (>60) white women, while increasing for African American women in all age groups [20]. Recently, both white and African American women have seen declines in breast cancer mortality rates, although the decline was smaller for African American women (1.6 % per year) when compared to white women (2.3 % per year) [6]. However, there are still disparities in 5-year survival rates between African American and white women (79 % vs. 92 %, respectively) [6].
11.3 Breast Cancer Subtypes
11.3.1 Introduction
Breast cancer is a heterogeneous disease with respect to its etiology, prognosis, and response to therapy. ER status and tumor grade were among the first important prognostic and predictive factors. In the early 1970s, the first reliable assay for testing ER status in tumors was available [21]. Presence of ER is important for response to specific treatments (e.g., tamoxifen, aromatase inhibitors) and prognosis, and more recently may define etiologic subtypes. Additionally, breast cancer can also be characterized by menopausal status at time of diagnosis and by histologic subtype.
Recently, there have been large-scale efforts to further characterize the distinct genetic and genomic variation of breast tumors [22–24]. It was not until the early 2000s that four major breast cancer molecular subtypes were identified: luminal A-like, luminal B-like, HER2+ type, and triple negative (or basal-like) identified through gene expression profiling and hierarchical clustering analyses. As with ER status, these breast cancer subtypes are associated with different etiologies, risk factors, clinical outcomes, and treatment options (Table 11.1) [25]. Large-scale epidemiologic studies have utilized immunohistochemical staining for markers including ER, progesterone receptor (PR), and human epidermal receptor 2 (HER2) (can also be assessed through fluorescent in situ hybridization), epidermal growth factor receptor (EGFR) and cytokeratin (CK) 5/6 as a proxy for the gene expression [22]. Below is an overview of these breast cancer subtypes as summarized by the 2013 St. Gallen Consensus report [22].
Table 11.1
Summary of breast cancer subtypes, molecular markers, prevalence, and clinical characteristics
Subtype | Markers | Prevalence (%) | Clinical characteristics |
|---|---|---|---|
Luminal A-like | ER+ and/or PR+/HER2−/low grade | 42–59 | Less aggressive, slow growing, endocrine sensitive. Low recurrence rates |
Luminal B-like | ER+ and/or PR+/HER+ Or ER+ and/or PR+/HER2-/high grade | 10–20 | Aggressive, poor-prognosis, less estrogen sensitive. High recurrence rates. High recurrence rates |
HER2+ type | ER-/PR-/HER2+ | 10–20 | Aggressive, poor short-term prognosis, more common in younger women. High recurrence rates |
Triple negative/Basal-like | ER−/PR−/HER2− | 10–20 | Fast growing, aggressive, often has a higher grade, and tends to metastasize. High recurrence rates. More common in African Americans, premenopausal women, and those with the BRCA1 mutations |
11.3.2 Luminal A-like
Luminal A-like breast cancer is the most common breast cancer subtype accounting for 42–59 % of all breast cancer cases and includes ER+/PR+/HER2-cancers [26]. These cancers are low grade 1 or 2 and/or have low Ki-67 expression (proliferative marker). Luminal A-like breast cancer tends to be less aggressive, slow growing, and more endocrine sensitive, thus associated with better prognosis [7]. Endocrine therapy is the primary treatment method and recurrence rates for women diagnosed with luminal A-like tumors are low [22].
11.3.3 Luminal B-like
Luminal B-like cancer account for 10–20 % of breast cancer [7]. Similar to luminal A-like breast cancer, most luminal B tumors are ER+/PR+. However, luminal B-like breast cancer usually has high expression of Ki-67 and includes both HER2+ and HER2−. For luminal B-like HER2− cancer, Ki-67 is high and PR is negative or low, while luminal B-like HER+ exhibit a wide range of Ki-67 and PR levels. Treatment for luminal B-like tumors incorporates estrogen therapy with chemotherapy and anti-HER2, as these have a worse prognosis, are more aggressive, and less endocrine sensitive. Furthermore, luminal B-like HER2-breast cancer has a high recurrence rate [22].
11.3.4 HER2+ Type (Nonluminal) or Erb-B2 Overexpressing
HER2+ type of breast cancer is defined by ER−/PR−/HER2+, where HER2 is over expressed and ER and PR are absent. HER2+ type of breast cancer accounts for approximately 10 % of all breast cancers [7]. These cancers tend to be more aggressive and are associated with a poorer short-term prognosis. Chemotherapy and more recently anti-HER2 therapy are used in treatment. The anti-HER2 targeted treatment regimens have substantially improved prognosis associated with this subtype [7].
11.3.5 Triple Negative/Basal-like
Triple negative breast cancer is defined by ER−/PR−/HER2−, and represents a diverse group of tumors. Basal-like cancers occur in about 10–20 % of breast cancer, are more common in African American women, premenopausal women, and those with BRCA1 mutations, and are associated with poorer short-term prognosis [7]. In epidemiologic studies, triple negative tumors can be further classified as basal-like if they express either CK5/6 and/or EGFR. The only standard treatment option available for triple negative cancer is chemotherapy [22].
11.4 Breast Cancer Risk Factors
11.4.1 Introduction
Epidemiological studies have convincingly established a number of risk factors for breast cancer. Many of these are reproductive factors during the course of a woman’s life. A unifying concept of these risk factors is that ovarian hormones initiate breast development and that monthly menstrual cycles induce regular breast cell proliferation. Puberty is an important period during breast development and is marked by a surge of hormones that induce regular breast cell proliferation. Pregnancy is also associated with higher circulating hormone levels, and is associated with a transient short-term increased risk of breast cancer. However, pregnancy and lactation are also associated with terminal differentiation of breast tissue and are associated with a reduced risk of breast cancer long term. The monthly pattern of cell division associated with regular menstrual cycles terminates with menopause, as indicated by cessation of ovulation and menstrual periods. The integration of pathology data into epidemiological studies of breast cancer has been the key, as the etiology of breast cancer based on molecular subtypes varies. The section below summarizes established breast cancer risk factors, as well as how these risk factors relate to specific breast cancer subtypes. Table 11.2 provides a summary of the established risk factors as well as the strength of the association overall and by ER/PR status [27–67]. Additionally, although the data are more limited, we have also summarized the current state of knowledge as it relates to risk factors and intrinsic subtypes in Table 11.3.
Table 11.2
Summary of risk factors for breast cancer, strength of association, and association with hormone receptor status
Comparisons | Overall risk ratio | Association by hormone receptor (HR) status | |
|---|---|---|---|
Age at menarche | 1 year delay | 0.95 [28] | Association evident for both HR+ and HR− |
Parity | Nulliparous versus parous | 1.2–1.7 [27] | Association evident for HR+, and maybe HR− |
Breastfeeding | Each year woman breastfeeds | 0.93–0.96 [29] | Association evident for both HR+ and HR− |
Oral contraceptives | Longer duration | Association evident for both HR+ and HR− | |
Menopausal hormone therapy | Longer duration (5+ years vs. never) | Association evident only for HR+ | |
Early-life adiposity | per 1-unit increase | 0.88–0.91 [41] | Association evident for both HR+ and HR- |
BMI (only premenopausal) | 2 unit increment in BMI | 0.9 [42] | Association evident for both HR+ and HR− |
Weight gain since age 18 | Gained 25 kg after age 18 versus those that remained withing 2 kg of weight since they were 18 | 2.0 [43] | Association evident only for HR+ |
Physical activity | Recent total physical activity (≥27 MET vs. <3 MET) and total physical activity (active or moderately active vs. not active) | Association evident for both HR+ and HR− | |
Family history | Increasing number of affected relatives (1, 2, or 3+ vs. no family history) | Association evident for both HR+ and HR- | |
Alcohol | Increases with 1 drink per day | Association evident only for HR+ | |
Age at menopause | Increase in risk per increase in year | 1.03 [30] | Association evident only for HR+ |
Mammographic density | >75 % vesus <5 % | 4.64 [60] | Association evident for both HR+ and HR− |
Circulating estrogen (postmenopausal) | Increasing quartiles (all levels vs. lowest) | Association evident only for HR+ | |
Circulation androgens (postmenopausal) | Increasing quintiles (highest Q vs. lowest Q) | Association evident only for HR+ |
Table 11.3
Summary of association between breast cancer risk factors by subtype and direction of association
Risk factors | ||||||||
|---|---|---|---|---|---|---|---|---|
Older age at menarche | Higher parity | Breastfeeding | Oral contraceptives | Higher BMI in premenopausal women | Weight gain since age 18 | Family History | Alcohol | |
Luminal A-like | − | − | − | − | − | + | + | + |
Luminal B-like | − | ‡ | − | ‡ | + * | + | + | ‡ |
HER2+ type | ‡ | ‡ | ‡ | ‡ | ‡ | ‡ | + | + |
Triple Negative/ Basal-like | − | + | − | + | + | ‡ | + | ‡ |
11.4.2 Age at Menarche
Later age at menarche has been consistently associated with a lower risk of breast cancer [27]. Risk of cancer decreases by 5 % for every 1-year delay in the start of menarche [28]. Brinton et al [68] observed a 23 % lower breast cancer risk in women who started menstruating after the age of 15 when compared to women who started menstruation at 12 years or younger. More recent results from the large pooling efforts of the Collaborative Group on Hormonal Factors in Breast Cancer [69] are consistent with previous findings of a dose–response relationship between age at menarche and risk of breast cancer.
Earlier menarche may be associated with earlier onset of regular ovulation menstrual cycles, which leads to a greater lifetime exposure of endogenous hormones [70]. The relation between age at menarche and breast cancer may be modified by menopausal status. A pooled analysis across studies found that each additional year in delay of menarche was associated with a stronger 9 % decrease in premenopausal breast cancer compared to a 4 % decrease in postmenopausal women [71]. The relationship between age at menarche and breast cancer also varies by molecular subtype. Although earlier age at menarche is inversely associated with both ER+/PR+ and ER−/PR− breast cancers, the magnitude of effect is greater for hormone receptor positive cancers [72]. In a systematic review of 39 studies, older age at menarche was consistently associated with moderately decreased risk of triple negative breast cancer [73]. These results were further confirmed by a population-based study that found that increases in age at menarche (per 2 years) are inversely associated with risk of basal-like breast cancer (Odds Ratio (OR) 0.8, 95 % Confidence Interval (95 % CI) 0.7–0.9) [74]. Further supporting these results, Millikan et al. [75] found that earlier-onset menarche (age < 13 years old) increased the risk of basal-like breast cancer (OR 1.4, 95 % CI 1.1–1.9). Older age at menarche appears to also decrease risk for luminal A-like and luminal B-like breast cancer [73]. There is no clear relationship between age at menarche and between HER2+ breast cancer.
11.4.3 Parity and Age at First Birth
Parity is often defined as the number of times a women has had a full-term pregnancy or pregnancy lasting >20 weeks [76]. In general, nulliparous women have increased risk of breast cancer compared with parous women [27]. However, the strength of association depends on the age of a woman’s first birth; a younger age at first term pregnancy is associated with a lower lifetime risk of breast cancer [27]. Research suggests that the protective effect of pregnancy takes 10–15 years to manifest [77]. In fact, there is a transient increase in risk of breast cancer for the first ten years after pregnancy [78, 79]. The dual effects of pregnancy on breast cancer are attributed to the proliferation of breast cells during pregnancy which may lead to growth of mutated cells (increasing risk) as well as the differentiation of mature breast cells making them less susceptible to carcinogens (decreasing risk). A higher number of births have also been consistently associated with a reduced risk of breast cancer [80]. Some studies also suggest that more closely spaced births are more protective for breast cancer.
The relationship between parity varies by breast cancer subtype. Greater parity is associated with a reduced risk for luminal A-like cancer [73], while it may increase risk for triple negative/basal-like cancer [75, 81–85]. There is no clear relationship between parity and HER2+ or luminal B-like breast cancer [73]. Younger age at first birth was also associated with decreased risk of luminal A-like and luminal B-like cancer, with stronger protective effect for luminal-A cancer in women with greater parity [83–85].
11.4.4 Breastfeeding
Longer breast feeding duration (≥6 months) has consistently been associated with a reduced risk of breast cancer. There is also strong evidence that the relationship is dose-dependent and is independent of parity. In a pooled analysis of 47 studies from 30 countries (N = 50,302 cases and N = 96,973 controls), the relative risk of breast cancer is reduced by 4.3 % (95 % CI 2.9–5.8 %) for each year that a women breastfeeds and by 7.0 % (95 % CI 5.0–9.0 %) for each birth [29]. After adjusting for parity, the relative risk for ever versus never having breastfed was 0.96 (p = 0.04) [29]. The two main mechanisms by which lactation may reduce risk of breast cancer are: (1) delaying regular ovulatory cycles, and (2) further terminal differentiation of breast tissue.
Longer breastfeeding duration is protective for luminal A-like, luminal B-like, and triple negative (or basal-like) breast cancer [75], with inconclusive results for HER2+ type [73]. Additionally, lactation may mitigate the increased risk of ER−/PR− breast cancer subtypes associated with parity [86]. The relationship between breast feeding and basal-like tumors is one of the most consistent protective factors for basal-like tumors and represents an opportunity for preventing this aggressive breast cancer subtype.
11.4.5 Oral Contraceptives
The hypothesis that oral contraceptive use increases the risk of breast cancer was first proposed several decades, and more than 50 studies to date have investigated the association.
Most epidemiological studies find no significant increase in breast cancer risk associated with ever use or duration of use and risk of breast cancer [30]. However, current and recent users of oral contraceptives have a small increased risk of breast cancer compared with never users (Relative Risk (RR) 1.2, 95 % CI 1.2–1.3) [30], and the increased risk appears to be no longer evident within 10 years after stopping use of oral contraceptives. Longer durations of use in young women <35 years old appear to increase breast cancer risk [31, 32], however it is unclear if this relationship is confounded by the recency of use. Additionally, age at first use of oral contraceptives may play a role in breast cancer risk [30].
It is important to note that majority of data in epidemiologic studies contributing to the literature and large pooled analyses are based on early formulations of oral contraceptives that had higher doses of ethinyl estradiol and different types of progestins than are currently available [87, 88]. It is noteworthy that the patterns, dose, and combination of use of oral contraceptives have varied over time. Since the 1960s, age at initiation of oral contraceptive has decreased, duration of use has increased while the doses have decreased. Most oral contraceptives contain a combination of ethinyl estradiol and a progestin. In the 1960s, the ethinyl estradiol dose in oral contraceptives was ≥100 mg. Today, the ethinyl estradiol dose is around 20–30 mg [87, 88]. Additionally, the formulations have changed over time with at least nine different progestins. There is limited long-term data evaluating the currently available formulations and breast cancer risk. Additionally, progestin-only contraceptives, long-acting contraceptives, and implantable levonorgestrel (Norplant) have not been thoroughly investigated and more research is needed, as these are continuing to grow in popularity.
Relatively few studies have evaluated the association between oral contraceptives and breast cancer subtype. There is some evidence that oral contraceptives may be more strongly associated with risk of triple negative cancers than luminal A-like cancers [73, 84, 89]. In a recent systematic review [73], there was insufficient data on HER2+ and Luminal B-like cancers to understand their relationship with oral contractive use.
11.4.6 Menopausal Hormone Therapy
Menopausal hormone therapies containing estrogens have been used for over half a century, and over three dozen epidemiological studies, six meta-analyses, and one larger pooled analysis published over the past 30 years that investigate associations with breast cancer risk. Most studies have found that menopausal hormone therapy use increases breast cancer risk; however, the magnitude of risk depends on the formulations used and duration of use [33–39]. Meta-analyses have reported a 30–45 % increased risk of breast cancer with greater than five years of use compared with never use [33–38]. A large, prospective analysis in the Nurses’ Health Study [40] found that the increased risk of breast cancer was limited to women with current or recent use of menopausal hormone therapy. Risk increased with longer duration of current use (RRCurrent 5+ years versus never users 1.5, 95 % CI 1.2–1.8). In a large, pooled analysis of epidemiological studies, the association between current use of menopausal hormone therapy and risk of breast cancer was also strongest for those with the longest duration of use; RR for <1 years was 1.1, 1–4 years was 1.1, 1.2 for 5–9 years, 1.1 for 10–14 years, and 1.6 for 15+ years [39].
A woman’s body weight may play a role in the association of menopausal hormone therapy and breast cancer risk. The magnitude of association between menopausal hormone use and risk of breast cancer appears to be higher among women who are leaner compared with heavier women [39]. This different effect of hormone therapy by BMI is consistent over many studies including the Women’s Health Initiative, a randomized control trial [90]. Further, for women who quit using menopausal hormones, the increased risk of breast cancer decreases and is similar to never users after quitting for 5 or more years, regardless of their duration of use [39].
Estrogen only and estrogen plus progestin (E&P) menopausal hormone therapies are both associated with increased risk in breast cancer, with a slightly higher risk seen in women who use E&P compared to estrogen only. Increases in breast cancer risk were seen in the Breast Cancer Detection and Demonstration Project [91] with recent use of estrogen only (RR 1.2, 95 % CI 1.0–1.4) and E&P (RR 1.4, 95 % CI 1.1–1.8). Similarly, the Million Women’s Study [92] observed increased breast cancer risk for current users of preparations containing estrogens only (RR 1.3, 95 % CI 1.2–1.4), and a higher risk for E&P (RR 2.0, 95 % CI 1.9–2.1). However, results from the Million Women’s Study suggested little differences in the associations between specific estrogens and progestins, doses or regimen types (e.g., sequential vs. continuous) [92]. Menopausal hormone therapy use appears to be associated with an increased risk of ER+ breast cancers, but not ER-cancers [93]. Given the large body of consistent evidence, the International Agency for Research on Cancer (IARC) has classified estrogen plus progestin therapy as a human carcinogen [94].
A number of studies in the U.S. and globally have reported declines in breast cancer incidence rates after 2002 [8, 95–99], the year that the Women’s Health Initiative trial published their results on the positive association between E&P therapy and increased breast cancer risk [100, 101]. Following this publication, the prescribing pattern for menopausal hormone therapy dramatically declined in the U.S. Although based on ecologic data, the relatively rapid declines in breast cancer incidence, specifically hormone receptor positive cancers, mirrored declines in menopausal hormone therapy prescriptions suggesting that the decline in incidence is attributable to reduced exposures to combined hormone therapies [8, 99, 102].
11.4.7 Body Size Throughout the Life Course
11.4.7.1 Introduction
The relationship between adiposity and breast cancer risk varies over the life course. In general, there is consistent evidence that adult premenopausal BMI is inversely related to risk of premenopausal breast cancer, while postmenopausal BMI is positively associated with postmenopausal breast cancer risk. More recent studies have provided consistent evidence that adiposity early in life (e.g., childhood and adolescence) is inversely associated with breast cancer risk. Interestingly, this protective effect of early-life body size is associated with both premenopausal and postmenopausal breast cancer. Below we discuss early-life body adiposity premenopausal BMI, postmenopausal BMI, and weight gain in relation to breast cancer risk.
11.4.7.2 Early-Life Body Size
There is consistent evidence that body fatness during childhood and adolescence is associated with a reduced risk of breast cancer, with the effect lasting throughout lifetime [43, 103]. In the Nurses’ Health Study II cohort [104], women who were heavier at ages 5 and 10 had half the risk of premenopausal breast cancer compared to those who were leanest at these ages [41]. Similarly, there is an inverse association between BMI at age 18 (or 20) and breast cancer observed in a number of other countries and racial/ethnic groups [105–109]. Additionally, the strong inverse association is observed for both ER+ and ER− breast cancers [41, 106]. Few studies have investigated early-life body size and breast cancer based on molecular subtype. Results from the Carolina Breast Cancer Study show that women who reported being heavier than their peers in 5th grade had a nonsignificant reduced risk of basal-like breast cancer (OR 0.5, 95 % CI 0.2–1.4) [75]. The mechanisms by which adiposity early in life may reduce breast cancer risk are not well understood. A few potential mechanisms have been suggested including that girls who are overweight may have slower sexual maturation, slower pubertal growth [110], and more anovulatory cycles [111].
11.4.7.3 Premenopausal and Postmenopausal BMI
Prospective studies [43, 112] and meta-analyses [42] have found an inverse relationship between adult body weight and incidence of premenopausal breast cancer. In a recent meta-analysis [42], the relative risk was 0.94 (95 % CI 0.92–0.95) for a two unit (kg/m2) increase in BMI in premenopausal women. One hypothesis is that heavier premenopausal women have more irregular menstrual cycles and increased rates of anovulatory infertility, thus decreasing risk due to fewer ovulatory cycles and less exposure to ovarian hormones [113].
Although there is an inverse association between BMI and premenopausal breast cancer, there is only a weakly positive relationship or no association observed in postmenopausal women [112, 114, 115]. The lack of a strong association appears to be due to the influence of the protective effects of early pregnancy and lasting protective effects of overweight early in life [43, 103].
11.4.7.4 Weight Gain
Weight gain in adulthood has been positively associated with increasing breast cancer risk in postmenopausal women. After menopause, the main source of circulating estrogens is the adipose tissue. Therefore, a higher body fat percentage after menopause translates to a higher woman’s exposure to estrogen. After menopause, obese women have both higher levels of endogenous estrogen and higher risk of breast cancer. An increased breast cancer risk is also seen in individuals who gain 25 kg after age 18; women who gained 25 kg had two times the risk of breast cancer compared to women who maintained their weight within two kg [43]. Weight gain since age 18 has consistently been associated with ER+ breast cancer subtypes (luminal A and luminal B-like) [73]. Although the data are more limited, there is a suggestion that weight gain since age 18 may also be associated with basal-like cancer [75].
11.4.8 Physical Activity
Epidemiological evidence suggests a possible relationship between physical activity and breast cancer risk, specifically in postmenopausal women. Results from one of the first pooled studies [116] (two case-control studies) on physical activity and breast cancer observed a significant inverse association between total physical activity and breast cancer risk (OR 0.9, 95 % CI 0.8–1.0) as well as specifically leisure time activity (OR 0.8, 95 % CI 0.7–0.9) for the highest quintile versus the lowest quintile [116]. Most recent studies from large, prospective studies also observe inverse associations for postmenopausal breast cancer. The European Prospective Investigation into Cancer (EPIC) cohort observed inverse associations with household and recreational physical activity [44]. Total physical activity was also associated with a decreased risk of breast cancer (Hazard ratio (HR)active vs. inactive 0.87, 95 % CI 0.79–0.97; HRmoderately active vs. inactive 0.92, 95 % CI 0.86–0.99) [44]. Additionally, there was a suggestion that the inverse association was strongest for ER+/PR+ tumors. In the Nurses’ Health Study [45], postmenopausal women who engaged in higher amounts of recent total physical activity also had a lower breast cancer risk (HR 0.9, 95 % CI 0.8–0.9; ≥27 MET [approximately 1 h/day of brisk walking] versus <3 MET (<1 h/week walking). In this study, there was no evidence that the association varied by hormone receptor status.
The modest inverse association between physical activity and breast cancer appears to be limited to hormone receptor positive cancers in most but not all studies. In the pooled case-control study described above [116], leisure time activity since age 50 was inversely associated with ER+/ PR+ cancers but not other subtypes. Recent results from EPIC [44] also observed the strongest association between recreational and household activity and ER+/PR+ tumors (HR 0.84, 95 % CI 0.74–0.96, active vs. inactive) and total physical activity (HR 0.88, 95 % CI 0.78–0.99, moderate active vs. inactive), in line with results from the NIH-AARP Diet and Health Study [117]. Total weekly energy expenditure from recreational physical activity (modest-low intensity) was inversely associated with ER+ breast cancer.
11.4.9 Family History
Family history is a well-established and strong risk factor for breast cancer. Results from the Nurses’ Health Study [46] suggest that the age-adjusted risk ratio was 1.8 (95 % CI 1.5–2.0) for women with a maternal history of breast cancer versus women who did not have a family history of breast cancer. The strength of the association with family history is stronger for women whose mothers were diagnosed with breast cancer at younger ages. For example, women whose mothers were diagnosed before the age of 40 had a more than twofold greater risk (RR 2.1, 95 % CI 1.6–2.8). In contrast, for women whose mothers were diagnosed at age 70 years or older, the RR was 1.5 (95 % CI 1.1–2.2) [46]. In a pooled study by Pharoah et al. [47], the relative risk estimates compared to women with no family history were: for any relative (RR 1.9, 95 % CI 1.7–2.0), first degree relative (RR 2.1, 95 % CI 2.0–2.2), mother (RR 2.0, 95 % CI 1.8–2.1), sister (RR 2.3, 95 % CI 2.1–2.4), daughter (RR 1.8, 95 % CI 1.6–2.0), mother and sister (RR 3.6, 95 % CI 2.5–5.0), and second-degree relative(RR 1.5, 95 % CI 1.4–1.6). The Collaborative Group on Hormonal Factors in Breast Cancer [48] found that the risk ratio increased with increasing number of affected relatives, with RR for one, two, and three or more affected relatives of 1.8 (99 % CI 1.7–1.9), 2.9 (99 % CI 2.4–3.6), and 3.9 (99 % CI 2.0–7.5), respectively [48]. Results from a systematic review observed that a positive family history was associated with a 1.5- to two-fold increased risk of breast cancer for all subtypes compared with women without a family history of breast cancer [73].
Genetic epidemiology studies have sought to uncover the extent to which inherited genetic factors underlie the strong family history. The proportion of breast cancer estimated to be due to rare highly penetrant genetic mutations such as BRCA1 and BRCA2 is small, approximately 3 [118]–10 % [119]. Hereditary syndromes such as Li-Fraumeni syndrome and Cowden syndrome are associated with increased risk of breast cancer. Li-Fraumeni syndrome is due to germline mutations in the p53 gene [120], while Cowden syndrome is due to germline mutations of the PTEN gene [121]. The cumulative lifetime risk of breast cancer in BRCA1 and BRCA2 carriers is estimated to range from 50 to 85 % [119]. These highly penetrant genetic mutations are rare in the population and account for less than 25 % of familial breast cancer risk [122, 123].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree