Tumor
Number
Female (%)
White (%)
Black (%)
Hispanic (%)
Asian (%)
Other (%)
Meningioma
25,946
73
69
12
11
7
0
Pituitary
11,921
55
57
17
18
7
0
Glioblastoma
10,854
42
80
6
10
5
0
Nerve sheath
5932
52
74
6
10
10
1
Astrocytoma
3089
45
73
7
14
6
0
Other
2996
51
67
11
16
6
0
Mixed/unclassified glioma
1936
42
67
10
17
6
0
Hemangioma/hemangioblastoma
1571
53
69
11
12
8
0
Lymphoma
1464
43
63
10
17
10
0
Oligodendroglioma
1064
49
72
3
14
9
1
Ependymoma
1054
50
66
6
22
7
0
Pilocytic astrocytoma
1018
46
63
13
19
4
1
Glioneuronal
814
46
67
12
15
5
0
Medulloblastoma
664
38
54
9
30
5
1
Craniopharyngioma
557
51
49
19
18
13
1
Choroid plexus
168
47
62
9
19
11
0
Pineal
150
57
66
24
7
2
0
Chordoma/chondrosarcoma
50
57
71
0
29
0
0
Approximately one-third of primary CNS tumors are located iÅn the meninges, while the supratentorium, ventricle, cerebellum, brainstem, cranial nerves/cauda equina, pituitary gland, and pineal gland account for 23, 1, 3, 2, 7, 3, and 14 % of tumors, respectively [7]. Tumors in other parts of the CNS comprise the remainder [7]. Patient age significantly impacts the distribution of tumors of the CNS. Among patients 0–4 years old, embryonal tumors/medulloblastoma is the most common tumor of the CNS, while between the ages of 5–14 years, 15–34 years, and 35 years and above pilocytic astrocytoma, pituitary adenoma, and meningioma are the most common, respectively [7]. Histopathologic classification of CNS tumors show a diverse range of diagnoses [8], most of which are relatively rare, making epidemiologic associations difficult. This chapter will therefore focus on two of the more common tumors of the CNS, meningioma and glioblastoma.
15.2 Meningioma
As noted above, meningioma represents the most common intracranial brain tumor [1, 2]. Meningioma originate from the dura that surround the brain and spinal cord, often within the sites of reflection (falx cerebri and tentorium cerebelli). The most important prognostic factor in patients with meningioma is grade (see Fig. 15.1). World Health Organization (WHO) grade I meningioma are the most common type (Fig. 15.2) and are associated with very high cure rates following surgical resection. Many patients with presumed grade I tumors discovered incidentally on imaging may need no treatment at all. Historical series suggest that approximately 7 % of patients presenting with meningioma have WHO grade II (i.e., atypical) lesions [9], while more modern series, which incorporate the 2000 and 2007 WHO pathologic reclassification systems for meningioma, suggest that an even higher proportion may be atypical [10, 11]. WHO grade III (malignant) meningioma represents the rarest of the three entities, accounting for only a few percent of cases. The prognosis for patients with malignant meningioma is relatively poor.
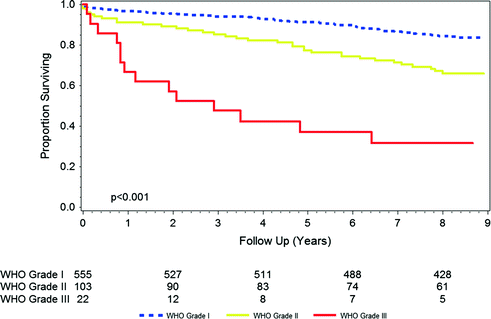
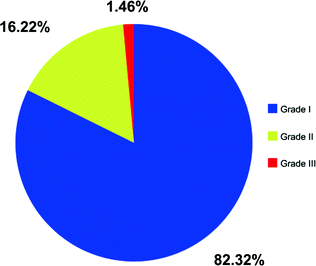

Fig. 15.1
Overall survival by grade in patients diagnosed with meningioma in 2003. Source SEER

Fig. 15.2
Distribution of WHO grade among patients with newly diagnosed meningioma in 2012. Source SEER
15.2.1 Symptoms
Meningioma are often asymptomatic. Many are diagnosed incidentally, typically for imaging obtained for other reasons [1]. When symptomatic, presenting symptoms may be nonspecific (headache, nausea, seizures) or may correlate with the specific region of involvement of the underlying brain. Frontal lobe lesions are associated with mental status changes and weakness, while dominant temporal lesions can cause aphasia. Cerebellar tumors often cause ataxia and gait disturbances. Occipital lesions can impact vision while parietal lesions can cause problems with higher order sensorimotor function. Lesions in the skull base can be associated with cranial nerve palsies, and spinal lesions can cause back pain, numbness, weakness, gait disturbances, and rarely bowel/bladder incontinence. Larger tumors near narrow portions of the ventricular system can cause obstructive hydrocephalus.
15.2.2 Diagnosis
Meningioma can be diagnosed via characteristic findings on diagnostic imaging, such as contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI). On CT some meningiomas can calcify, and meningioma typically take up contrast. On MRI meningioma are located extra axially, are typically isointense on T1 precontrast imaging, and become hyperintense in a typically homogeneous manner with contrast administration. Contours are typically smooth and well-defined. A dural tail may be present, but dural tails can be seen in other entities as well, such as lymphoma, sarcoidosis, and chloroma.
15.2.3 Management
Small, incidentally discovered meningioma can typically be monitored with surveillance imaging, as the growth rate is often 1 mm/year or less, and some lesions may never grow [12, 13]. Treatment for symptomatic lesions, large lesions, or observed lesions which grow significantly in patients with otherwise favorable prognoses entails maximal safe resection as a first step. The impact of extent of resection on survival and recurrence in patients with meningioma has been most notably demonstrated by Simpson for benign meningioma, with emphasis on the negative prognostic influence of trace residual disease in the dural tail or venous sinuses [14]. Apart from grade, extent of resection represents the most important prognostic factor for patients with meningioma [15–17].
Adjuvant management typically depends on the grade and extent of resection. WHO grade I lesions that are gross totally resected should be observed, and observation is reasonable for many subtotally resected lesions as well. Whether to offer radiation therapy to patients with gross totally resected WHO grade II meningioma remains controversial, but several series support a local control benefit with radiation in such settings [18–20]. Generally, patients with subtotally resected WHO grade II meningioma and all patients with WHO grade III meningioma should receive radiation, given the very high rates of recurrence in such populations. Systemic therapy has marginal efficacy and is usually reserved for multiply recurrent lesions. Hormonal agents, chemotherapy, platelet-derived growth factor inhibitors, angiogenesis inhibitors, epidermal growth factor inhibitors, somatostatin analogs, interferon, and other agents have been tested in patients with advanced/recurrent meningiomas, often with disappointing results [21–23].
15.3 Epidemiology of Meningioma
15.3.1 Prevalence and Incidence
As discussed above, meningioma are very common among the general population, with MRI studies indicating that 1–2 % of the population harbors a meningioma [1, 2]. Vernooij et al. [1] as part of the Rotterdam Scan Study, prospectively performed thin-slice (1.6 mm), 1.5 T MRIs of the brain in approximately 2000 individuals in the general population with a mean age of 63 years. The study found a prevalence of meningioma of 0.9 %; ranging in size from 0.5 to 6.0 cm [1]. Notably, contrast was not given, and scans were initially read by radiology or neurology residents and abnormal findings were reviewed by neuroradiologists. Both schema may have led to underestimation of the true prevalence of meningioma. The incidence of meningioma has been estimated to be 7.8/100,000 per year, although the rate in men (2.9/100,000) is significantly lower than in women (13.0/1000,000) [24]. Many are incidentally found.
15.3.2 Mortality
Most meningioma will have minimal clinical impact. Detailed population-based estimates for mortality rates are lacking. WHO grade I meningioma are typically cured with surgery alone, with radiation typically reserved for recurrent disease. Death due to a WHO grade I meningioma is rare. WHO grade II tumors, and especially WHO grade III tumors, carry a more significant mortality risk (Fig. 15.1), warranting aggressive treatment with maximal surgical resection and often adjuvant radiation therapy.
15.3.3 Risk Factors
15.3.3.1 Sex
Most meningioma occur in women (Table 15.2). In the Rotterdam Scan Study, the prevalence of meningioma varied by gender (1.1 % in women; 0.7 % in men). Autopsy studies have also shown that meningioma more commonly occur in women, by a factor of up to 3:1 [25]. Among patients diagnosed with a meningioma in 2012, per the SEER database, 73 % were female [6]. Notably, the female preponderance was primarily for WHO I tumors; patients with WHO III tumors were 50 % female. Spinal meningioma may be especially more likely to occur in females as well.
Table 15.2
Gender distribution among patients with WHO grade I, II, and III meningioma, SEER data, 2004–2012
Meningioma grade | Male (%) | Female (Number, %) |
|---|---|---|
WHO I | 28 | 72 |
WHO II | 44 | 56 |
WHO III | 50 | 50 |
15.3.3.2 Race
Meningioma may be slightly more common in patients of African American race, relative to white race. Rates in Asian Americans may be lower. Aggregated data from Central Brain Tumor Registry of the U.S. (CBTRUS)/SEER from 2006 to 2010 reveal an age-adjusted incidence (as stratified by race) of 7.2/100,000 for whites, 8.8/100,000 for African Americans, and 5.1/100,000 for Asian Americans. Rates in Hispanic Americans appear to be similar to whites, with an incidence of 7.3/100,000 [7].
15.3.3.3 Age
The risk of meningioma appears to increase with age. In the Rotterdam Scan Study, the prevalence of meningiomas increased from 0.5 % in those age 45–59 years to 1.6 % in persons 75 years of age or older. Autopsy studies have shown that the risk of meningioma indeed increases with age [25]. Among patients diagnosed with a meningioma in 2012 per the SEER database, 1, 6, 30, 43, and 20 % were age <20, 20–39, 40–59, 60–79, and ≥80 years of age, respectively [6]. There does not appear to be an association between age and grade of meningioma, per the SEER data.
15.3.3.4 Neurofibromatosis Type 2
Neurofibromatosis type 2 is an autosomal dominant condition that predisposes patients to the development of multiple intracranial neoplasms such as vestibular schwannomas (often bilateral), ependymal tumors, and intracranial/spinal meningioma. Patients can often present with cataracts, retinal hamartomas, and cutaneous/subcutaneous tumors. The condition is caused by a mutation in the NF2 gene, which codes the protein merlin, a tumor-suppressor gene. Estimates of prevalence have ranged from 1:25,000 to closer to 1:100,000 [26–28]. When meningioma are present in a patient with neurofibromatosis type 2, they are often multiple. Meningioma in such patients often develop by the patient’s early 20s, with increasing prevalence as age increases. Most patients will develop meningioma should they live to late adulthood, and meningioma in this population are more likely to be atypical or malignant [29, 30].
15.3.3.5 Ionizing Radiation
Ionizing radiation represents one of the strongest risk factors for the development of meningioma, particularly when given in childhood. The latency period between receipt of radiation and development of meningioma is often long. Taylor et al. [31] followed approximately 13,000 patients in Great Britain diagnosed with cancer when they were less than 15 years of age, between 1940 and 1991. The most common intracranial tumor that developed was a meningioma. The risk of meningioma increased strongly, linearly, and independently with dose of radiation to meningeal tissue. Those whose meningeal tissue received 0.01–9.99, 10.00–19.99, 20.00–29.99, 30.00–39.99, and ≥40 Gy had risks (of development of meningioma) that were twofold, eightfold, 52-fold, 568-fold, and 479-fold, respectively, as compared to those whose meningeal tissue was unexposed to radiation. Of note, receipt of intrathecal methotrexate was also linked to the development of meningioma in this study [31]. The Childhood Cancer Survivor Study, which followed patients treated for pediatric malignancies, concluded that radiation exposure was significantly associated with the development meningioma (Odds Ratio (OR) 9.94, 95 % Confidence Interval (CI) 2.17–45.6) [32]. Other studies of patients treated with therapeutic radiation involving the CNS have also identified higher than usual rates of development on meningioma in patients, often with a significant latency of years—decades [33].
Patients exposed to radiation for reasons other than cancer also appear to be predisposed to development of meningioma. Follow up of patients treated with radiation as part of the therapeutic regimen for tinea capitis display higher rates of meningioma, with a long latency from the delivery of radiation to the development of meningioma [34]. Atomic bomb survivors have similarly displayed higher rates of development of meningioma, also in a dose dependent manner [35]. Whether the lower doses associated with medical/dental imaging can contribute to the development of meningioma is more controversial. Several studies have examined this issue and there is some evidence for a correlation [36–38]. However, whether more modern imaging techniques, which often use lower doses, are associated with meningioma is more debatable. Unfortunately, radiation-induced meningioma tend to be more aggressive than sporadic meningioma [39, 40]. The higher incidence of meningioma after radiation to the CNS as well as the potentially poorer prognosis associated with such tumors should promote clinicians to minimize diagnostic and therapeutic radiation exposure to patients.
15.3.3.6 Hormones and Body Mass Index
Meningioma tumors may contain progesterone, estrogen, and androgen receptors. Benson et al. [41] recently conducted a meta-analysis of published studies evaluating the relationship between menopausal hormonal therapy and the development of meningioma. The authors found that estrogen increased the risk of meningioma (Relative Risk (RR) 1.31, 95 % CI 1.20–1.43) relative to patients who did not use estrogen, but combined estrogen–progestin hormonal treatment did not (RR 1.05, 95 % CI 0.95–1.16). Additional evidence linking hormones and development of meningioma relates to higher rates of meningioma in obese patients, given the link between adiposity and hormonal levels [42, 43].
15.4 Glioblastoma
Glioblastoma represents one of the most common intraparenchymal tumors of the CNS. The prognosis for patients with glioblastoma is extremely poor [4, 5] and long-term survival is unfortunately uncommon. With aggressive treatment in favorable prognosis patients, 2–10 % of patients may survive five years or longer. Although treatment advances have been made, durable treatment options for patients with glioblastoma remain lacking.
15.4.1 Symptoms
Unlike patients with meningioma, most patients with glioblastoma present to medical attention as a result of symptoms. When symptomatic, presenting symptoms may be nonspecific (headache, nausea, mental status changes, and seizures) or may correlate with the specific region of involvement of the underlying brain. In addition, the invasive and sometimes diffuse nature of glioblastoma often results in compromise of performance status and reduced ability to complete activities of daily living.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree