- Coeliac disease is an intestinal mucosal disorder caused by hypersensitivity to gluten present in several cereal grains, such as wheat, barley, rye, and possibly oats. Symptoms may range from severe malabsorption to oligosymptomatic cases, often with extradigestive symptoms (anaemia, osteopaenia), which are particularly frequent in adults.
- The only effective treatment for coeliac disease is the absolute withdrawal of gluten from the diet for life. This is particularly important to minimise the risk of late complications, such as intestinal lymphoma and other gastrointestinal malignancies.
- Tropical enteropathy and tropical sprue are a group of disorders mainly affecting the small intestine of individuals living in, or visiting, the tropics. Correction of water and electrolyte imbalances and nutritional replacement are the basis for the management of tropical sprue.
- Protein–energy malnutrition and other micronutrient deficiencies are frequent in patients with inflammatory bowel disease (IBD) and result from a combination of poor nutrient intake, increased metabolic demands, increased intestinal protein losses, and nutrient malabsorption.
- If patients with IBD are severely malnourished or suffering from severe inflammatory bouts of the disease, they will need to be treated with artificial nutrition.
- Many patients with irritable bowel syndrome (IBS) believe that their symptoms are triggered by specific foods, but this is difficult to prove scientifically. However, specific sugar malabsorptions should be investigated, by means of the hydrogen breath test, in those patients in whom diarrhoea and/or bloating continue to occur.
11.1 Introduction
The majority of the diseases of the gastrointestinal tract are not fatal but are significant causes of poor health, responsible for large proportions of patients attending hospital and local medical services. The major conditions associated with the gastrointestinal tract are summarised in Box 11.1.
Infectious diseases of the gastrointestinal tract are covered in Chapter 21, pancreatitis in Chapter 13, and liver disease in Chapter 12. Management of food allergy is covered in Chapter 8.
Dysphagia
Dysphagia (difficulty in swallowing) is a common consequence of many different types of illness or injury, resulting in mechanical or neurological impairment of the swallowing process. Swallowing occurs in two phases – oropharyngeal and oesophageal. In the oropharyngeal phase, food in transferred from the mouth via the pharynx to the upper oesophagus. The oesophageal phase carries food from the pharynx to the stomach. Some of the most common causes of dysphagia are listed in Table 11.1.
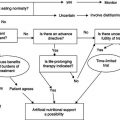
Attempting to swallow foods or liquids without the ability to do so carries a high risk of aspiration pneumonia and is potentially fatal. If dysphagia is suspected, it is very important that the patient’s swallow is assessed by a health professional qualified to do so. Dysphagia is almost always accompanied by a reduced food intake, leading to significant weight loss, compromised immune function, and a high risk of dehydration. The primary aims of management of dysphagia are listed in Box 11.2.
- diarrhoea and vomiting;
- dyspepsia;
- peptic and duodenal ulcers;
- constipation, abdominal pain, and irritable bowel;
- haemorrhoids and anal fissure;
- dysphagia;
- hernia;
- gallstones;
- appendicitis;
- malabsorption syndromes;
- ulcerative colitis and Crohn’s disease;
- diverticular disease of the colon;
- pancreatitis;
- liver disease;
- food intolerance.
Table 11.1 The most common causes of dysphagia.
| Oropharyngeal | |
| Neuromuscular | Stroke Head injury Muscular disorders Motor neuron disease Parkinson’s disease |
| Physical obstruction | Pharyngeal pouch Goitre |
| Psychological | Globus hystericus |
| Infections | Tonsillitis |
| Oesophageal | |
| Neural | Achalasia Multiple sclerosis Diffuse oesophageal spasm |
| Muscular | Scleroderma Dystrophia myotonica |
| Physical obstruction | Stricture: cancer chronic oesophagitis Diverticulum External compression (aortic aneurysm) Postoperative |
| Infections | Candida |
Gastro-oesophageal reflux disease
Gastro-oesophageal reflux disease (GORD) occurs as a normal event, and clinical features only occur when antireflux mechanisms fail enough to allow gastric contents to make prolonged contact with the lower oesophageal mucosa. The presence of acid and enzymes irritates the mucosa and causes pain, and repeated injury may cause mucosal damage and inflammation (oesophagitis). This in turn increases the risk of oesophageal adenocarcinoma. The causes of GORD are listed in Box 11.3.
- Assess the nature of the swallowing problem.
- Determine a safe and adequate feeding route.
- Determine the appropriate texture and consistency of foods and fluids.
- Meet nutritional needs.
- Ensure adequate hydration status.
- Educate patient and/or carers.
- Monitor progress and ensure continuity of care.
- oesophageal sphincter weakness;
- increased pressure within the stomach;
- high pressure from the abdominal area (obesity or pregnancy);
- hiatus hernia.
- Eat smaller meals more frequently.
- Avoid eating late at night.
- Avoid bending, lifting, or lying down after meals.
- Reduce weight if overweight.
- Avoid excessive consumption of caffeine-containing drinks and alcohol.
- Avoid highly spiced foods or those foods known to exacerbate symptoms.
About 50% of patients can be treated successfully with simple antacids. Other need proton-pump inhibitor drugs. Antireflux surgery may be indicated in patients where medical treatment is insufficient. Loss of weight and raising of the head of the bed at night are caadjuvant measures in some patients. Some dietary methods of managing GORD are summarised in Box 11.4.
Cancer of the oesophagus and stomach
Surgical treatment of cancer of the oesophagus may be partial or total oesophagectomy, oesophagogastrectomy, or partial or total gastrectomy, depending on the site and extent of the cancer. Since many oesophageal cancer patients may be severely nutritionally compromised, preoperative and early postoperative nutritional support is often necessary (see Chapter 9). Weight loss, iron-deficiency anaemia, B12 deficiency, and osteomalasia may all occur after gastric surgery and must be identified and managed.
Short-bowel syndrome
Short-bowel syndrome occurs after small-bowel resection where less than 1 m of the bowel remains. The management of short-bowel syndrome patients depends on the extent of bowel resection, the site of the resection, and the presence or absence of the ileocaecal valve. Intestinal adaptation can occur postoperatively, leading to hypertrophy of the mucosal surface and lengthening of the villi, leading in turn to a substantial increase and recovery of the absorptive surface.
The fact that gastrointestinal disease is a major cause of malnutrition for patients both in hospital and in the community is not surprising given its pivotal role in digestion and absorption. In some conditions, such as coeliac disease and some food allergies, the illness is caused by the intolerance of some nutrient, and treatment is based on the withdrawal of the offending foodstuff. In others, such as gastrointestinal infections and tropical enteropathy, the causative agent is not dietary, but the consequences of the illness are nonetheless nutritional and metabolic in nature and need to be managed.
In other conditions, such as inflammatory bowel disease (IBD) (ulcerative colitis and Crohn’s disease), where the aetiology is unknown, not only may nutritional support provide macro- and micronutrients, but certain elements may play a pharmacological role in the management of the disease itself. Finally, in functional intestinal diseases, dietary management may play a role in alleviating or reducing symptoms.
This chapter will focus on the nutritional consequences and management of some non-neoplastic diseases of the small bowel and the colon. These include:
11.2 Coeliac disease
Coeliac disease – also termed coeliac sprue or gluten-sensitive enteropathy – is a disease of the small bowel characterised by:
Epidemiology
The true prevalence of coeliac disease is probably unknown, since many patients have mild or no symptoms, and in these cases the disease often remains undiagnosed. In most European countries, the prevalence of the disease ranges from 0.05 to 0.2%. Some studies report an increased incidence of coeliac disease in recent decades. This must be due, at least in part, to an increasing awareness of the disease and the recognition of oligosymptomatic patients. Classically, coeliac disease used to seem to be less frequent in the USA. However, recent studies of serological screening in blood donors suggest that the frequency is the same as that in Europe.
Although some areas – including Scandinavia, the British Isles, and the Mediterranean basin – are particularly at risk, coeliac disease is considered a worldwide phenomenon. However, it is rare in Africa, East Asia, and the Caribbean.
Pathogenesis
Coeliac disease is a hereditary illness. Its development is strongly associated (90–95% of cases) with the human leukocyte antigen (HLA) haplotype DQw2 on chromosome 6. However, environmental factors also play a role in the development of the disease.
The most important environmental factor for developing coeliac disease in susceptible individuals is gluten, a protein component of several cereal grains. Some alcohol-soluble fractions of gluten, termed prolamines, are particularly harmful. These are α, β, γ, and Ω gliadins (from wheat), hordeins (from barley), secalins (from rye), and possibly avidins (from oats). In contrast, rice and corn are not harmful to the intestinal mucosa.
Some factors may play a role in precipitating symptoms. These include gastrointestinal surgery, pregnancy, high-dose or early gluten challenge, and viral infection. Breast-feeding seems to delay the age of onset of coeliac disease, but there is no clear evidence that it reduces the lifetime risk for developing the disease.
There is now evidence that coeliac disease is an intestinal immunological disease. In brief, selected prolamin peptides are presented by distinctive surface-HLA heterodimers to specific T cells. T-cell activation then results in a cascade of proinflammatory cytokines. At the same time, B cells and plasma cells produce antibodies that stimulate the release of other noxious mediators, possibly causing cell-mediated cytotoxicity. As a result of this process, the intestinal mucosa is damaged.
Coeliac disease may occur in association with other immune-based diseases (Box 11.5). Individuals suffering from these conditions should be screened for coeliac disease. Amongst these associated diseases, dermatitis herpetiformis deserves special mention. This is an itchy bullous skin rash that involves the extensor surfaces of the limbs, trunk, and scalp, characterised histologically by the deposition of IgA granules in the dermal–epidermal junction. The disease is accompanied by intestinal damage, sometimes asymptomatic, but indistinguishable from that seen in coeliac disease. In fact, both the intestinal and cutaneous lesions regress with dietary gluten withdrawal. Thus, dermatitis herpetiformis is at present considered part of the spectrum of gluten sensitivity rather than a mere associated condition to coeliac disease.
Magnitude of the problem: the spectrum of gluten sensitivity
Coeliac disease involves the mucosa of the small bowel, while the submucosa, muscularis, and serosa are usually unaffected. Proximal segments of the bowel (duodenum and jejunum) are damaged more frequently, while ileal involvement is less frequent. The mucosal damage in coeliac disease varies from almost normal morphology (latent coeliac disease) to the classical picture of villous atrophy and ‘flattened mucosa’.
- dermatitis herpetiformis;
- type I diabetes mellitus;
- autoimmune thyroid disease;
- selective IgA deficiency.
- autoimmune connective-tissue disease;
- Sjögren syndrome;
- IgA nephropathy;
- IBD;
- sclerosing cholangitis;
- primary biliary cirrhosis;
- Down’s syndrome.
Traditionally, coeliac disease has been considered a paediatric disease, but it is also frequently diagnosed in adult life. Classical symptoms in children include diarrhoea, vomiting, anorexia, irritability or apathy, and failure to thrive. In adults, the ‘complete’ coeliac syndrome consists of chronic diarrhoea, weight loss, malabsorption and iron-deficiency anaemia. In most severe cases, the so-called ‘coeliac crisis’ would ensue if left untreated – with tetany, haemorrhagic diathesis, and oedema – constituting a true gastrointestinal emergency.
In recent decades it has become evident that coeliac disease may present with only scarce and/or mild symptoms. This is particularly true in the adult. Diagnosis of the disease may thus be delayed. Digestive symptoms can include recurrent abdominal pain in children and complaints of indigestion and bloating in adults. A number of patients have only extradigestive symptoms. Some of the most frequent manifestations, which may occur (alone or in combination) in the absence of the classic malabsorptive syndrome in coeliac disease, are listed in Box 11.6. Coeliac disease may remain clinically silent for years despite the existence of histological lesions. This situation has been reported to occur in first-degree relatives of coeliac patients and other risk groups.
- unespecific dyspepsia (recurrent abdominal pain, bloating, etc.);
- iron-deficient anaemia, refractory to oral iron therapy;
- low bone-mineral density, osteoporosis, and its consequences (particularly if excessive for the age);
- peripheral neuropathy;
- intellectual deterioration, epilepsy with posterior cerebral calcifications;
- dental-enamel hypoplasia;
- recurrent oral aphtous ulcerations;
- amenorrhoea, delayed menarche, female infertility;
- repeated miscarriages;
- male impotence and/or infertility;
- arthritis and other joint symptoms;
- ‘unexplained’ mild–moderate increase in serum liver enzymes.
Diagnosis and screening
The diagnosis of coeliac disease is based on a demonstration of the characteristic histological findings in a well-orientated jejunal biopsy specimen, followed by clinical remission when the patient is put on a gluten-free diet. In 1990, the European Society of Paediatric Gastroenterology and Nutrition (ESPGAN) revised its diagnostic criteria, stating that a single jejunal biopsy could be enough for the diagnosis of coeliac disease. The old ESPGAN criteria required a second biopsy after withdrawing gluten from the diet (which must be normal), followed by a third one after gluten challenge (where histological lesion must reappear). At present a demonstration of normalised histology following a gluten-free diet is no longer required for a definitive diagnosis of coeliac disease.
Based on very high sensitivities and specificities, the best available tests are the IgA antihuman tissue transglutaminase (TTG) and IgA endomysial antibody immunofluorescence (EMA) tests, which appear to have equivalent diagnostic accuracy (TTG is the specific protein that is identified by the IgA-EMA). Antigliadin antibody (AGA) tests are no longer routinely recommended because of their lower sensitivity and specificity.
Serological screening should be made in patients with clinical suspicion of coeliac disease, in first-degree relatives of coeliac patients, or in individuals suffering from any of the associated conditions to the disease (Box 11.5). However, it must be stressed that serological tests do not replace intestinal biopsy in the diagnosis of coeliac disease. Moreover, patients with malabsorptive symptoms or signs must undergo jejunal biopsy irrespective of the results of the serological tests, in order to rule out an enteropathy other than coeliac disease. Negativisation of antibodies can be used as a monitoring test for compliance to the gluten-free diet.
Treatment
Coeliac disease is perhaps the one disease above all other gastrointestinal disorders in which diet is the key to management. Patients with coeliac disease and/or dermatitis herpetiformis must adhere to a strictly gluten-free diet for life. Cutaneous manifestations of dermatitis herpetiformis usually respond to treatment with sulphones (dapsone), but such a therapy fails to reverse the intestinal disease. A major reason for recommending absolute removal of gluten from the diet is prevention of late development of malignancies. Coeliac patients have been found to have an increased risk for malignancy – particularly T-cell lymphoma of the small bowel, oesophageal cancer, and cancer of the oropharynx – as compared to the general population. A strict gluten-free diet for life decreases such a risk, although unfortunately this is not fully eliminated in the case of lymphoma.
Avoiding gluten is easier to say than to do. Follow-up studies indicate that only 50–70% of coeliac patients maintain a strict gluten-free diet later in life. Noncompliance is more frequent in children and, particularly, in teenagers. Removal of obvious sources of the offending grains (wheat, rye, barley, and oats), such as bread, breakfast cereals, pasta, cakes, and pastry, is relative easy. However, hidden sources of gluten are frequent, as wheat flour is added in many manufactured food products. Adequate food labelling is very important in this setting. Lists of foods which obviously contain gluten (and are thus forbidden), are positively gluten-free (and thus permitted), and may be surreptitious sources of gluten (to be considered with caution) are provided in Table 11.2.
The institution of an effective gluten-free diet requires extensive education of the patient by their physician, as well as the advice of an experienced professional dietitian. Recipe books are also available in some countries. In addition, patients should be encouraged to contact and join the coeliac societies or other support groups existing in many countries, which produce up-to-date lists of brands of gluten-free foods.
Table 11.2 Food chart for coeliac patients.
| Forbidden (obviously contain gluten) | Permitted (obviously gluten-free) | Surreptitiously contain gluten (not to be eaten unless explicitly labelled as gluten-free by the manufacturer) |
| Bread and flour from wheat, rye, barley, and oatsa Cakes, pastry, biscuits, pies, and other baked goods made with these flours Italian pasta (spaghetti, macaroni, etc.), wheat semolina Manufactured products with the above flours (flans, custard, ice-cream, jelly, etc.) Malted foods (e.g. malted milk) Drinks containing cereals (beer, ale) | Milk and other dairy products Any kind of fresh meat, fish, or other seafood Eggs Rice, corn, millet, buckwheat, sorghum, and any foodstuff made with flour from these cereals Tapioca, soybean Fruits, vegetables, potatoes Butter, margarine, oils, and other fats Salt, pepper, vinegar Sugar, honey Coffee made with ground coffee beans, tea, and other herbal infusions Homemade cakes and pastry without the offending flours | Manufactured foodstuffs which might contain cereal flours as additive, thickening, or flavouring agents Sausages, pâtés, luncheon meats, canned meats, and poultry Meat sauces (soy, Worcestershire, etc Cheese spreads Salad dressings, mustard, ketchup, tomato sauce, etc. Instant and canned soups, bouillon cubes Instant coffee and tea Candy bars, chocolate mixes As a rule, any canned food Any food possibly contaminated with flour during harvesting, packaging, storage, or in the kitchen Residual gluten in ‘gluten-free’ wheat starch used in baked products Nonfood items with trace amounts of gluten Excipients of some medications (either prescriptions or over-the-counter) Communion wafers Grain-derived alcoholic drinks (whisky, vodka, etc.) Anything with misleading labelling |
aSome patients can probably tolerate certain amounts of oats without risk (see text).
Commercially available brands of gluten-free foods (e.g. pasta, biscuits, bread) help to diversify the diet of coeliac patients. These brands are internationally identified with a special logo (Figure 11.1). The Codex Alimentarius defines a food as gluten-free when ‘…the total nitrogen content of the gluten containing cereal grains used in the product does not exceed 0.05 g/100 g of these grain on a dry matter basis’. It is important to note that this norm does not refer to the minimal amount of gluten tolerated by a coeliac patient. In some particularly susceptible patients, even this small amount could induce insidious symptoms. In addition, gluten-free brands are expensive. Therefore, they should be used merely as a complement of the coeliac diet, which must be essentially based on naturally occurring gluten-free foods.
In recent years, there has been increasing evidence to suggest that some coeliac patients can tolerate moderate amounts of oats. The source of oats must be free of gluten contamination (in harvesting, storing, or packaging) in order to be safe, and it is uncertain whether larger and long-term oats challenges would be harmless in these patients. Thus, if a coeliac patient is going to consume oats, a pure source should be used, and monitoring for relapse should be maintained.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree