2Leipzig University Medical Centre, Germany
- In chronic liver disease (CLD), patient malnutrition is frequent and an independent negative prognostic factor. Protein depletion in particular is associated with loss of vital physiological functions and impaired liver function. Malnutrition can be detected by simple clinical methods.
- In CLD, patients’ glycogen stores are depleted and therefore even short periods of fasting are noxious and must be avoided. In general, spontaneous food intake is overestimated and in fact is very often totally insufficient.
- In CLD, trace element and vitamin deficiencies are frequent. Measures to prevent refeeding syndrome or Wernicke’s encephalopathy must be adopted.
- In CLD, the goal of nutrition therapy is to ensure the adequate provision of energy, protein, and micronutrients, making use of oral nutritional supplements, tube feeding, or parenteral nutrition.
- Nutrition therapy improves the liver function, body composition, morbidity, and mortality of CLD patients.
- CLD patients require a protein provision higher than that recommended for healthy, well-nourished individuals. In encephalopathy, especially after gastrointestinal haemorrhage, the use of branched-chain amino acid-enriched solutions is beneficial.
- Obese patients with nonalcoholic steatohepatitis (NASH) benefit from sustained weight reduction, irrespective of the medical strategy (dietary counselling, orlistat, bariatric surgery, etc.) by which this is achieved.
- In acute liver failure (ALF), the treatment goals are to ensure: (i) the adequate provision of energy, and especially ensuring euglycaemia by giving glucose, lipid, vitamins, and trace elements; and (ii) optimal rates of protein synthesis, by providing an adequate intake of protein or amino acids. In the majority of patients, these goals can be achieved by enteral nutrition.
- In many respects (energy requirement, utilisation of exogenous glucose and lipids, use of early enteral nutrition), ALF patients are not different from critically ill patients suffering from other entities. Hypoglycaemia is a frequent problem in ALF, meriting particular attention.
- Irrespective of the modality of nutrition (enteral, parenteral), tight metabolic monitoring is warranted in order to avoid cerebral oedema of ALF.
12.1 Introduction
Nutrition has long been recognised as a prognostic and therapeutic determinant in patients with chronic liver disease (CLD), and was therefore included as one of the variables in the original prognostic score proposed by Child and Turcotte. Nutritional status, however, was not included in the widely used modified Child–Pugh score, and not all hepatologists consider nutrition issues relevant in the management of their patients. In this chapter, the scientific and evidence base of nutrition management of patients with liver disease is reviewed, in order to give recommendations for nutrition therapy.
12.2 Nutritional risk in liver-disease patients
Adequate nutrition is a complex process, which in healthy organisms is regulated in an adaptive response according to the prevailing condition. Therefore, the assessment of nutritional risk of patients must include variables indicative of the physiologic capabilities – the nutritional status – and the burden inflicted by the ongoing or impending disease and/or medical interventions. A meaningful assessment of nutritional status should encompass not only body weight and height, but also information on energy and nutrient balance, as well as body composition and tissue function, reflecting the metabolic and physical fitness of the patient facing a vital contest. Such information can best be interpreted when viewed dynamically (e.g. weight loss over time).
Numerous descriptive studies have shown higher rates of mortality and complications, such as refractory ascites, variceal bleeding, infection, and hepatic encephalopathy (HE), in cirrhotic patients with protein malnutrition, as well as reduced survival when such patients undergo liver transplantation. In malnourished cirrhotic patients, the risk of postoperative morbidity and mortality is increased after abdominal surgery.
In cirrhosis or alcoholic steatohepatitis (ASH), poor oral food intake is a predictor of increased mortality. In nutrition-intervention trials, patients with the lowest spontaneous protein intake showed the highest mortality. Dietary intake should be assessed by a skilled dietitian, and a three-day dietary recall can be used in outpatients. Appropriate tables for food composition should be used for the calculation of proportions of various nutrients. As a gold standard, the energy content of food servings and leftovers can be measured by bomb calorimetry.
Simple bedside methods such as the ‘Subjective Global Assessment’ (SGA) or anthropometry have been shown to identify malnutrition adequately. Composite scoring systems have been developed based on variables such as actual/ideal weight, anthropometry, creatinine index, visceral proteins, absolute lymphocyte count, delayed-type skin reaction, absolute CD8+ count, and hand grip strength. Such systems, however, include unreliable variables such as plasma concentrations of visceral proteins and 24-hour urine creatinine excretion, and do not confer an advantage over SGA.
The accurate measurement of nutritional status is difficult in the presence of fluid overload or impaired hepatic protein synthesis (e.g. reduced synthetic rate of albumin in cirrhosis). Sophisticated methods are required to assess body cell mass (BCM) by total body potassium counting, lean body mass by dual-energy X-ray absorptiometry (DXA), total body protein by in vivo neutron activation analysis (IVNAA), or total body water by isotope-dilution techniques. Among bedside methods, the measurement of phase angle α or determination of BCM using bioimpedance analysis is considered superior to methods such as anthropometry and 24-hour creatinine excretion, despite some limitations in patients with ascites.
Muscle function is reduced in malnourished CLD patients and, as monitored by hand grip strength, is an independent predictor of outcome. Plasma levels of visceral proteins (albumin, prealbumin, retinol-binding protein) are heavily influenced by hepatic synthetic capacity, alcohol intake, or acute inflammatory conditions. Immune status, which is often considered a functional test of malnutrition, may be affected by hypersplenism, abnormal immunological reactivity, and alcohol abuse.
12.3 Effect of nutritional state on liver disease
Under-nutrition
Severe under-nutrition in children can cause fatty liver, which in general is fully reversible upon refeeding. In children with kwashiorkor, there seems to be a maladaptation associated with less-efficient breakdown of fat and oxidation of fatty acids compared to children with marasmus. An impairment of fatty acid removal from the liver could not be observed. Under-nutrition impairs specific hepatic functions, such as phase-I xenobiotic metabolism, galactose elimination capacity, and plasma levels of c-reactive protein in infected children. In nutrition-intervention trials in cirrhotic patients, quantitative liver-function tests improved more, or more rapidly, in the treatment groups. This included antipyrine, aminopyrine, and ICG clearance, as well as galactose elimination capacity. It is unknown whether fatty liver of malnutrition can progress to CLD.
Quantitative liver-function tests can be used to monitor the effects of nutritional intervention on liver function. They are not useful, however, for the identification of patients who will benefit from nutritional intervention, since none of the tests can differentiate whether loss of liver function is due to reduced hepatocellular mass or lack of nutrients. A simple test is needed that can distinguish between these two alternatives, in analogy to the intravenous (IV) vitamin K test, in order to estimate the potential benefit of nutritional support in individual patients.
Over-nutrition
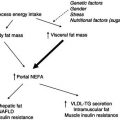
In obese humans subjected to total starvation, weight-reducing diets, or small-bowel bypass, the development of transient degenerative changes with focal necrosis was described in the 1970s. Nonalcoholic steatohepatitis (NASH) was initially described in weight losing-individuals; currently insulin resistance and obesity are its most common causes. It is estimated that in Europe, 20% of the population with moderate or no alcohol consumption has nonalcoholic fatty liver (NAFL), of which 20% progress to NASH. Analyses of dietary habits in NASH patients do not show a uniform pattern. Increased consumption of fat and n-6 fatty acids and increased consumption of carbohydrate and energy have been observed. The occurrence of insulin resistance has been attributed to a high fructose consumption from corn syrup, as used in soft drinks. Body mass index (BMI) and total body fat are predictors for the presence of NASH in the obese, and in patients undergoing bariatric surgery NASH is diagnosed on average in 40%, with a range of 24–98%. The key role of obesity is illustrated by the observation that weight reduction, regardless of whether it is achieved by dietary counselling, bariatric surgery, or drug treatment, has the potential to ameliorate or even cure NASH.
12.4 Effect of liver disease on nutritional state
Acute liver disease
Minor or moderate acute liver disease induces the same metabolic effects as any disease associated with an acute-phase response. In more severe acute liver disease, such as acute liver failure (ALF), there are profound changes in carbohydrate and protein metabolism. The effect on nutritional status depends on the duration of the disease and the presence of an underlying CLD which might have already compromised the patient’s nutritional status. In ALF, in particular hyperacute liver failure, a so-far healthy individual is struck by a fulminant disease which leads to spontaneous recovery, liver transplantation, or death within days. On the other hand, patients with subacute liver failure are at considerable risk of becoming malnourished and suffer from severe changes in body composition during the protracted course of their illness.
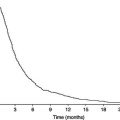
Figure 12.1 Protein depletion in patients with liver cirrhosis, as measured by in vivo neutron activation analysis. The graph shows the distribution of the protein index (ratio of measured to estimated pre-illness total body protein) in 268 patients with liver cirrhosis ( ) and in 386 healthy volunteers (
) and in 386 healthy volunteers ( ). Am J Clin Nutr 2007; 85: 1257–1266. Copyright © American Society for Nutrition.
). Am J Clin Nutr 2007; 85: 1257–1266. Copyright © American Society for Nutrition.
Cirrhosis
Mixed-type protein-energy malnutrition with coexisting features of kwashiorkor-like malnutrition and marasmus is commonly observed in patients with cirrhosis. The prevalence and severity of malnutrition are related to the clinical stage of CLD, increasing from 20% of patients with well-compensated disease up to more than 60% of patients with severe liver insufficiency. Patients with cirrhosis frequently suffer from substantial protein depletion (Figure 12.1), and the resulting sarcopenia is associated with impaired muscle function and survival. Recovery from this loss in BCM can be achieved by the control of complications such as portal hypertension and by adequate nutrition. The aetiology of liver disease per se does not seem to influence the prevalence and degree of malnutrition and protein depletion, and the higher prevalence and more profound degree of malnutrition in alcoholics obviously results from unhealthy lifestyle and low socioeconomic conditions.
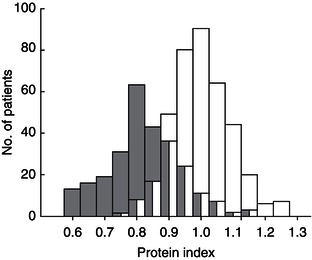
Figure 12.2 Loss of tissue function, exemplified by severe limitation of cardiopulmonary exercise capacity in cirrhotics. The graph shows the distribution of peak VO2 within the study population (n = 135). Peak VO2, expressed as percentage of predicted value, was normal (≥85%) in only 11.9% of cases and impaired in 88.1% of cases. Impairment was severe (<60%) in 54% of cases. With kind permission from Dharancy et al. (2008).
In hospitalised cirrhotics, fatigue, somnolence, and psychomotor dysfunction often lead to insufficient oral nutrition, even in the absence of overt HE.
Surgery and transplantation
After successful liver transplantation, in many patients there is an enormous weight gain in the first year following surgery and, unfortunately, a considerable number put their regained health in jeopardy by the development of full-blown metabolic syndrome. In the first year after transplantation, patients expand their body fat mass with no gain in lean body mass, and there is persisting impairment of non-oxidative glucose disposal in skeletal muscle. Even long-term survivors after liver transplantation exhibit an abnormal body composition with the phenotype of sarcopenic obesity. Preoperatively, cardiorespiratory exercise capacity is severely compromised in many cirrhotics (Figure 12.2), and this loss of physical function is associated with reduced survival after transplantation. The metabolic trauma of liver transplantation is associated with a loss in total body protein, irrespective of the nutritional management prior to the operation. This loss is associated with loss of tissue function, such as respiratory muscle function. There is growing evidence that in solid organ-transplanted patients, skeletal muscle deconditioning persists from the time of decreased physical performance prior to transplantation, which should be addressed by appropriate comprehensive rehabilitation programmes, including physiotherapy.
Taken together, these observations indicate that upon restoration of hepatic function and cessation of portal hypertension, full nutritional rehabilitation is possible and should be aimed at.
12.5 Pathophysiology and nutrient requirement in liver disease
Energy
Acute liver failure
In healthy individuals, hepatic energy expenditure contributes 25% to whole-body energy expenditure, and in ALF one would expect a reduction in oxygen-consuming processes like hepatic ketone body production and lactate elimination due to the loss of functioning hepatocyte mass. Indirect calorimetry in patients with ALF, however, showed an increase in resting energy expenditure (REE) of 18–30% when compared to healthy controls. Most likely, the accompanying systemic inflammatory-response syndrome has caused an increase in energy expenditure that more than outweighs the reduced oxygen consumption of hepatocytes. Thus, in terms of energy expenditure, patients with ALF are not different from critically ill patients with other aetiologies.
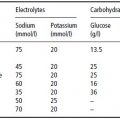
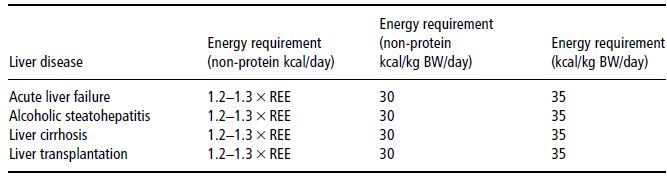
Table 12.1 Energy requirements in various types of liver disease, as calculated from measured or predicted resting energy expenditure (REE), on the basis of body weight, or as total energy requirement, including the energy content of amino acids or protein as given in recommended amounts.
Cirrhosis
On average, measured REE is of the same magnitude as energy expenditure predicted by the use of formulas (Harris–Benedict, Schofield, etc.). Likewise, in ASH patients, one study showed the same relationship between measured REE and predicted REE as in healthy individuals. Whenever available, indirect calorimetry should be used to measure REE, since in an individual patient measured REE may differ considerably from predicted values.
The question of hypermetabolism has been addressed in cirrhosis and ASH patients. ASH patients may be considered hypermetabolic when measured REE is related to their reduced muscle mass. Measured REE is increased by more than 20% above predicted REE in up to 35% of cirrhotic patients (hypermetabolism), and decreased by more than 20% below the predicted value in 18% of patients. In cirrhosis, hypermetabolism has been shown to be associated with reduced event-free survival and unfavourable outcome after transplantation, and seems to regress with improvement of body composition and after liver transplantation. For the diagnosis of hypermetabolism, however, indirect calorimetry is required, so that in daily practice most clinicians cannot use this approach.
Measurements of total energy expenditure indicate that the 24-hour energy requirement of cirrhotic patients amounts to about 130% of the basal metabolic rate (BMR) (Table 12.1). Diet-induced thermogenesis and the energy cost of defined physical activity in stable cirrhosis patients are not different from values obtained in healthy individuals. The spontaneous physical activity level, however, is considerably lower in cirrhotic patients. Obviously, the increased REE in advanced illness is balanced by diminished physical activity, reflecting the poor physical condition, and thus total energy expenditure is typically normal.
In cirrhotics without ascites, the actual body weight should be used for the calculation of the BMR when using formulas such as that proposed by Harris and Benedict. In patients with ascites, the ideal weight according to body height should be used, despite the suggestion from a series of 10 patients with liver cirrhosis, of whom only four were completely evaluated, in which it was suggested that ascites mass should not be omitted when calculating energy expenditure on the basis of body weight.
Surgery and transplantation
Liver-transplant patients on average have the same energy requirements as the majority of patients undergoing major abdominal surgery. In general, the provision of non-protein energy at 1.3 × REE is sufficient. In a longitudinal study, postoperative hypermetabolism peaked on day 10 after the transplantation at 124% of the predicted REE. By 6–12 months post-transplant, there was no longer a difference between the measured and predicted REE.
Carbohydrate metabolism
Acute liver failure
Hypoglycaemia is a clinically relevant and common problem in ALF, resulting entirely from loss of hepatic gluconeogenetic capacity, lack of glycogen, and hyperinsulinism. As a standard procedure, hypoglycaemia is treated by infusing glucose at a rate of 1.5–2 g/kg BW/day. Infection and cerebral oedema resulting from astrocyte swelling are the two key factors in the prognosis of ALF. Therefore, the rigorous control of blood glucose and tight metabolic monitoring may prove beneficial in this condition, where the central organ of metabolism is failing. Considering the facts that (i) glucose infusion is aimed at providing the critically ill with oxidative fuel essential for vital tissues such as CNS and erythrocytes, (ii) exogenous insulin at rates above 4 IU/hour cannot increase glucose oxidation, and (iii) in ALF there is insulin hypersecretion, hyperinsulinaemia, and insulin resistance, there seems to be little reason for insulin administration above 4 IU/hour in order to control glycaemia.
Cirrhosis
The utilisation of oxidative fuels is characterised by an increased rate of lipid oxidation in the fasting state and the frequent occurrence of insulin resistance (even in Child–Pugh class A patients). In the fasting state, glucose oxidation rate is reduced and hepatic glucose production rate is low despite increased gluconeogenesis due to a depletion of hepatic glycogen. Insulin resistance affects skeletal muscle metabolism: glucose uptake and non-oxidative glucose disposal such as glycogen synthesis are reduced, while glucose oxidation and lactate production are normal after glucose provision. It is not known to what extent glucose deposition as glycogen is impaired in skeletal muscle alone versus in both muscle and liver. Some 15–37% of patients develop overt diabetes, indicating an unfavourable prognosis.
Surgery and transplantation
In the early postoperative phase, there is often a disturbance of glucose metabolism, associated with insulin resistance. In this situation, hyperglycaemia should be managed by reducing glucose intake, because higher insulin doses are unable to increase glucose oxidation.
Fat metabolism
Acute liver failure
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree