The Epidemiology of Bladder Cancer
Jian-Min Yuan
Heather H. Nelson
INTRODUCTION AND OVERVIEW
One of the earliest established etiologic, exposure-cancer relationships was between exposures encountered during the manufacturing of synthetic dyes and subsequent bladder cancer development. There were hints of such a relationship by the late 1800s, strong anecdotal evidence by the early 20th century, supportive experimental evidence by the 1930s (that began to pinpoint the carcinogenic chemical exposure to specific arylamines), and strong systematic epidemiologic evidence by the early 1950s (1,2). Also in the 1950s, the first convincing epidemiologic evidence was published that cigarette smoking was by far the most important exposure contributing to bladder cancer development on a population basis (3,4). This relationship between smoking and bladder cancer relationship was over time shown to be a highly reproducible finding that extended across genders and many populations worldwide, with estimates from many recent studies indicating that smoking alone could explain half of all bladder cancer occurrences in most Western populations (5,6,7). This recognition, combined with a series of largely nonproductive major epidemiologic studies in the 1970s to establish other exposures as major contributors to bladder cancer development, such as artificial sweetener use and coffee drinking, has led to substantial neglect by epidemiologists in recent decades in developing any additional hypotheses or in conducting additional major studies to further understand the epidemiology and etiology of this important disease.
The epidemiology of bladder cancer is not, as one might predict, that of a disease whose main established risk factors are cigarette smoking and occupational exposure to arylamines. Although men are at substantially greater risk than women, as predicted from the epidemiology of cigarette smoking, white men have substantially higher rates than African American men (or for that matter higher than any other racial-ethnic group), which is not as predicted, nor is the strong positive relationship between socioeconomic status and risk. Moreover, there exist many populations around the world with high smoking rates, and consistent with these, high lung cancer rates, yet relatively low bladder cancer rates. These patterns of occurrence strongly suggest that there are still important additional, but as-yet-unidentified, risk factors, protective factors, or environmental-or genetic-risk modifiers that contribute to bladder cancer susceptibility.
The reason that cigarettes cause bladder cancer has not been definitively established, but it is generally believed that this association is due to small amounts of the same arylamines in cigarette smoke known to cause bladder cancer in occupational settings. Recent evidence suggests that use of permanent hair dyes might be a substantial population source of arylamines, as well as a substantial contributor to bladder cancer risk, especially among women. Recent work looking at probable important biomarkers of bladder cancer risk (i.e., arylamine adducts to hemoglobin) strongly suggests that there are additional important arylamines that contribute to bladder cancer risk, but the main source of exposure remains unknown. Even for established carcinogenic arylamines to the bladder, such as 4-aminobiphenyl (4-ABP), data suggest that major additional unknown sources of exposure exist. Further, data suggest that genetically determined modification of arylamine metabolism can affect individual bladder cancer risk, no matter what the underlying source of arylamine exposure.
In this chapter, we begin by reviewing the unique demographic patterns of bladder cancer, focusing especially on racialethnic variation in risk, socioeconomic characteristics, and bladder cancer risk patterns, and time trends in bladder cancer incidence over the past several decades. We then review in detail the epidemiologic and related evidence for the well-established bladder cancer risk and protective factors. We also review and summarize the epidemiologic evidence for some potential bladder cancer risk factors, which have been extensively studied but largely dismissed as significant contributors to bladder cancer occurrence. Finally, we discuss areas where there is clearly a need for additional research, including the current status of biomarker research and genetically determined risk modification.
DEMOGRAPHICS
Bladder cancer shows a close to 15-fold international variation in incidence. The highest rates occur among men in southern Europe with age-standardized rates (per world population) close to 40 per 100,000 people per year, followed by western and northern Europe and North America with annual incidence rates in the range from 20 to 30 per 100,000. Asians (including Chinese, Japanese, and Indians) are at low risk for bladder cancer, with annual age-standardized rates of around 3 to 10 per 100,000 in men and 1 to 3 per 100,000 in women (8).
Age, Sex, and Race
Bladder cancer currently ranks 6th in incidence among all cancers in the United States (4th in men and 12th in women) (9). The disease is rare prior to age 35, and two thirds of cases occur in people aged 65 or older. The huge excess in men is among the most prominent epidemiologic features of bladder cancer. Men overall have a fourfold excess of bladder cancer compared with women, and the male excess is observed to a somewhat comparable degree across all major racial-ethnic groups (8,9).
Bladder cancer is a disease for which non-Hispanic whites are at very high risk relative to other racial groups. In the
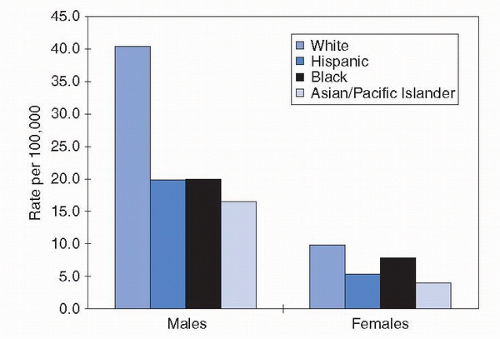
United States from 2002 to 2006, for example, the age-adjusted incidence rate of bladder cancer in non-Hispanic white men was more than twice that in black or Hispanic men. Asian-American men had rates that were lower still, roughly one third that of non-Hispanic white men (10). Women showed a somewhat comparable pattern by race ethnicity, albeit at substantially lower absolute-risk levels (Fig. 15.1).
United States from 2002 to 2006, for example, the age-adjusted incidence rate of bladder cancer in non-Hispanic white men was more than twice that in black or Hispanic men. Asian-American men had rates that were lower still, roughly one third that of non-Hispanic white men (10). Women showed a somewhat comparable pattern by race ethnicity, albeit at substantially lower absolute-risk levels (Fig. 15.1).
Social Class
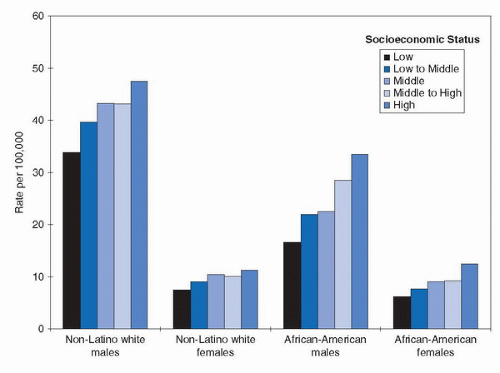
Some population-based cancer registries classify cancer patients according to social class characteristics based on their places of residence. Specifically, census information on income and/or educational levels of residents in the neighborhoods where cancer patients reside is used to rank cancer cases. In the Los Angeles County Cancer Registry, patients are placed into one of five social-class groupings. In general, high social class individuals, whether white or black or either sex, have a higher risk of bladder cancer (Fig. 15.2) (11).
Time Trends
Histopathology
The uroepithelial cells lining the human bladder can become transformed to tumor cells with different histopathologies. Approximately 95% of tumors of the urinary bladder in the United States are of transitional cell type. The remaining 5% include primarily squamous, glandular, or undifferentiated cell type tumors (8). In contrast, in Egypt and parts of the Middle East, squamous cell carcinoma of the bladder constituted up to 76% of all bladder cancer diagnoses in early 1970s, when infection with Schistosoma haematobium was endemic, and decreased to approximately one third of all confirmed bladder cancer cases in 2003 to 2007, reflecting decreasing prevalence of schistosomiasis (14).
Transitional cell carcinoma occurs in two distinct morphologic forms, which have different natural histories and prognoses. Almost two thirds of transitional cell bladder cancers diagnosed in the Western world are papillary noninfiltrating morphology (i.e., superficial cancer). The remaining transitional cell carcinomas invade the underlying stroma of the bladder (i.e., invasive cancer).
ENVIRONMENTAL EXPOSURES
Occupational Exposures
Although various industries and occupations have been linked to bladder cancer in epidemiologic studies, by far the most important, at least historically, is the synthetic dye industry. The first synthetic dye, aniline purple, made from aniline, an arylamine present in coal tars, was accidentally formulated in
the 1860s. By the late 1800s, synthetic textile dye production was a major industry. The first anecdotal reports of bladder cancer occurring among employees in these chemical dye workers were published in 1895, and many others in the United States and Europe followed. Experimental evidence that oral ingestion of 2-naphthylamine, an arylamine extensively used in the manufacturing of industrial dyes, could cause bladder cancer in dogs fueled further interest in a possible etiologic association (15).
the 1860s. By the late 1800s, synthetic textile dye production was a major industry. The first anecdotal reports of bladder cancer occurring among employees in these chemical dye workers were published in 1895, and many others in the United States and Europe followed. Experimental evidence that oral ingestion of 2-naphthylamine, an arylamine extensively used in the manufacturing of industrial dyes, could cause bladder cancer in dogs fueled further interest in a possible etiologic association (15).
Nonetheless, the first systematic epidemiologic evidence that arylamine-exposed chemical dye workers had a large and unequivocal increase in bladder cancer risk was not published until 1954 in London, by Case et al. (2). They observed approximately a 20-fold increase in bladder cancer risk in such workers compared to the general population and concurrently showed that rubber workers, another occupational group with extensive exposure to 2-napthylamine and to benzidene, a chemically related compound, also had substantial elevation in bladder cancer risk.
Other industries and occupations that appear to be at high risk of bladder cancer based on observations from multiple epidemiologic studies include the leather industry, commercial painters, hair dressers, truck drivers, and aluminum workers (16,17). In fact, many of these associations can be linked to workers in these occupations or industries with exposure to the same arylamines, which have been proven to be human bladder carcinogens in other settings.
Cigarette Smoking
The earliest epidemiologic studies on cigarette smoking and bladder cancer were conducted in the 1950s (3,4). Scores of such studies have provided data on this relationship, although not all were designed specifically for that purpose. Although epidemiologic methods and statistical analytic strategies have improved dramatically since those original studies and much more detail is now known about this relationship, the general conclusion of these first studies was correct: cigarette smokers, overall, increase their bladder cancer risk some two-to threefold compared to lifelong nonsmokers. This approximate increase in risk seems to extend across many populations around the world, including multiple racial-ethnic groups. There have been a number of reviews summarizing studies contributing to our understanding of these relationships, and we will not repeat that review here (18,19). Nonetheless, some additional generalizations can be made about this relationship. Risk of bladder cancer increases with increasing number of cigarettes smoked on a daily basis and with increasing duration of smoking, although it is unclear if this relationship is strictly linear. The risk of bladder cancer decreases after people quit their smoking habit, but there is no indication that the risk among former smokers returns to the level of a lifetime nonsmoker even many years after cessation (18). Although cigarette smoking clearly increases bladder cancer risk among both men and women, relative risk appears to be higher in women for a given dose and duration of smoking (20), a finding that is supported by biomarker data on smoking-related carcinogens (21). If inhalation patterns, tar content of cigarettes, or filters modify bladder cancer risk among smokers, the impact of these effects must be very modest as they are not easily detectable by epidemiologic methods. Finally, the impact of pipe and cigar smoking on bladder cancer risk appears to be substantially less than among cigarette smokers, whereas smokeless tobacco use does not appear to affect risk at all.
Although the reason that cigarette smoking causes bladder cancer is unproven, it very likely is due to the presence of carcinogenic arylamines in cigarette smoke, especially 2-naphthylamine and 4-aminobiphenyl (4-ABP), a potent bladder carcinogen (21). One of the most fascinating features of bladder cancer epidemiology is that, despite the clear importance of cigarette smoking in bladder cancer development as noted above, there exist a number of populations worldwide with high smoking rates and high lung cancer rates, but relatively low bladder cancer rates (18). In fact, even among different racial-ethnic groups with comparable smoking habits living in the same geographic area, there can be substantial variation in bladder cancer risk.
Bladder Cancer in Nonsmokers
At the most, only 50% of the bladder cancer burden in a given population can be attributed to tobacco smoking. The search for nonsmoking causes of bladder cancer was the goal of numerous studies launched in the last 40 years, and no credible hypothesis has emerged until recently. Using hemoglobin adducts of selected arylamines as biomarkers of exposure, the Los Angeles study demonstrates that among lifelong nonsmokers, bladder cancer risk increases with increasing exposure to 4-ABP, presumably from diffuse, as-yet-unidentified sources in the environment (22). Even more interesting is the finding that lifelong nonsmokers who develop bladder cancer are also exposed to higher levels of selected anilines that hitherto had not been linked to human bladder cancer (23).
Environmental tobacco smoke (ETS) exposure (“passive” smoking) is one of the sources for arylamines. Elevated levels of 4-ABP have been seen among lifelong nonsmokers with ETS exposure (24,25). Population-based studies that examined the relation of ETS exposure to bladder cancer risk generally reported null results (26,27). However, ETS exposure in childhood, especially for women, was associated with an elevated bladder cancer risk in several studies (28,29,30).
Hair Dyes
Hair dyes represent another substantial source of arylamine exposure in humans. In developed countries, including North America, Europe, and Japan, up to 40% of adult women use hair dyes on a regular basis. There are three major types of hair dyes: permanent dyes, semipermanent dyes, and temporary rinses. Permanent dyes are the most common, accounting for roughly three fourths of all hair dye use. Many commercial brands of hair dyes are mutagenic and some are known to contain recognized animal carcinogens (31).
Early studies of bladder cancer risk in hair dye users, conducted in the 1970s and 1980s, reported largely null results. Few of these studies differentiated among the three major types of hair dyes (permanent, semipermanent, or temporary rinses). In the recent Los Angeles study, personal use of hair dyes was assessed according to the types of hair dyes normally used. Women who reported regular and sustained use of permanent hair dyes were found to experience an increased risk of bladder cancer comparable in level to women who smoked. Risk increased with increasing frequency of hair dye applications and with increasing duration of use (32). The New Hampshire study found a similar, but statistically nonsignificant positive association between hair dye and bladder cancer in women (33). Among women who were smokers as well as permanent hair dye users, the level of increased bladder cancer risk was approximately the sum of the individual effects from smoking and use of permanent hair dyes (34). This latter statistical observation is compatible with the notion of a common etiologic pathway (i.e., arylamine exposure) linking these two seemingly distinct environmental exposures to bladder cancer development.
4-ABP may exist as a contaminant in commercially produced 1,4-phenylenediamine, a key component of many permanent hair dyes. A recent study detected the presence of 4-ABP (in parts per billion) in 8 of 11 commercial brands of hair dyes in the United States, and found nursing mothers who were current users of hair dyes to exhibit significantly elevated levels of 4-ABP DNA adducts in epithelial cells from breast milk relative to nursing mothers who were not currently using hair dyes (35).
Other Risk/Protective Environmental Factors
Although our understanding of bladder cancer epidemiology and etiology focuses around the important contribution of exposure from various sources to carcinogenic arylamines, a number of other potential risk or protective factors have also been extensively explored over the past several decades in relationship to bladder cancer occurrence.
Nonsteroidal Anti-inflammatory Drugs
Nonsteroidal anti-inflammatory drugs (NSAIDs) are recognized chemopreventive agents for colon cancer, presumably due to their inhibitory actions on the expression of cyclooxygenase 2, an inducible enzyme whose overexpression has been linked to most major forms of cancer, including bladder cancer. There are consistent experimental data indicating NSAIDs as inhibitors of carcinogen-induced bladder cancer in animals (36). Epidemiologic evidence shows that regular and sustained users of NSAIDs possess a lower risk of bladder cancer (37,38,39). The degree of protection seems to vary by class of chemical formulation, in general agreement with experimental findings in animals.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree