1. As a supplemental modality to screen for breast cancer in women at an elevated risk for breast cancer
2. Known breast cancer patient
(a) Staging of breast cancer to determine extent of disease to aid in treatment planning
(b) Monitoring patients undergoing neoadjuvant chemotherapy
3. As a supplemental modality to diagnostic mammography and/or ultrasound in:
(a) Diagnosis of occult breast cancer in patients metastatic axillary adenocarcinoma with an unknown primary
(b) Problem-solving assessment in:
(i) Lesion characterization
(ii) Sonographically occult one-view-only mammographic finding
4. To assess integrity of breast implants
Breast MRI as a Supplemental Screening Modality in Women with an Elevated Risk for Breast Cancer
The value of breast MRI in screening for breast cancer in women at an elevated risk has been shown in several observational studies [1–15]. Currently the American Cancer Society recommends annual screening with MRI for women with a 20–25 % lifetime risk of developing breast cancer [2] [Box 8.2]. There is insufficient evidence showing benefit in screening women with a 15–20 % lifetime risk, and MRI is not recommended for those with a less than 15 % lifetime risk of developing breast cancer [2]. Women with a genetic BRCA1 and BRCA2 mutations account for about 3 % of all breast cancers. These women and their untested relatives may have a 50–60 % lifetime risk of breast cancer. There is insufficient evidence for routine screening with MRI in women with a personal history of breast cancer, in those diagnosed with atypical ductal or lobular hyperplasia or DCIS, or in those with extremely dense breast. About 5–10 % of breast cancers are truly hereditary [3].
Box 8.2 American Cancer Society Guidelines for Breast Screening with MRI as an Adjunct to Mammography
Recommend annual MRI screening (based on evidence*) |
BRCA mutation |
First-degree relative of BRCA carrier, but untested |
Lifetime risk ~20–25 % or greater, as defined by BRCAPRO or other models that are largely dependent on family history |
Recommend annual MRI screening (based on expert consensus opinion) |
Radiation to chest between age 10 and 30 years |
Li-Fraumeni syndrome and first-degree relatives |
Cowden and Bannayan-Riley-Ruvalcaba syndromes and first-degree relatives |
Insufficient evidence to recommend for or against MRI screening |
Lifetime risk 15–20 %, as defined by BRCAPRO or other models that are largely dependent on family history |
Lobular carcinoma in situ (LCIS) or atypical lobular hyperplasia (ALH) |
Atypical ductal hyperplasia (ADH) |
Heterogeneously or extremely dense breast on mammography |
Women with a personal history of breast cancer, including ductal carcinoma in situ (DCIS) |
Recommend against MRI screening (based on expert consensus opinion) |
Women at less than 15 % lifetime risk |
Table 8.1 compares the sensitivity of mammography to breast MRI in screening for breast cancer in high-risk women. MRI is clearly the winner; mammography performs poorly mainly due to reduced sensitivity resulting from dense breast tissue that is more prevalent in these young women who are being screened. One of the initial large observational studies examining the benefits of screening for breast cancer in women at high risk was the Dutch Magnetic Resonance Imaging Screening study undertaken in 2004. Since most women at high risk refused consent for randomization, the study population was compared with a control group of non-screened women from an external source [4]. The multicenter study included 1,909 women of whom 358 were gene carriers, 1,052 had a lifetime risk of 30–50 % [high-risk group], and 499 women had a lifetime risk between 15 and 30 % [moderate-risk group]. There were 19 malignancies in mutation carriers, in the high-risk group there were 15 cancers, and in the moderate-risk group there were 11 cancers. The sensitivity of MRI in this study was 79.5 % and that of mammography was 33.3 %, clearly showing the superiority of breast MRI over screening mammography [4]. The Magnetic Resonance Imaging Breast Screening [MARIBS] trial was a prospective study of 649 high-risk women; in this study, mammography was shown to have a sensitivity of only 40 % compared to 77 % with breast MRI. Combined sensitivity for the two modalities was high at 94 %, justifying the use of both modalities to screen for breast cancer. The High Breast Cancer Risk Italian trial [HIBCRIT] included 278 women, all of whom were BRCA1 and BRCA2 carriers; the sensitivity of MRI was 94 % compared to 59 % with mammography. A recent large trial including 609 women demonstrated a sensitivity of 17 % for whole breast ultrasound, 33 % for film-screen mammography, 39 % for digital mammography, and 71 % for MRI. These studies have also shown the value of MRI in detecting cancer at a more favorable tumor stage. The Dutch trial showed that MRI-screened patients had a significantly higher percentage of small cancers, 10 mm or less in 43 % of women compared with 12.5 % in age- and risk-matched women, and had positive lymph nodes in 21.4 % of women compared to 56.4 % in the non-MRI-screened women. There is, therefore, indirect evidence of a beneficial effect on prognosis; however, in the absence of randomized clinical trials, it is not possible to reach conclusions regarding mortality rate reduction or even improved disease-free survival [1].
Table 8.1
Comparison of sensitivity of screening MRI and mammography for detection of breast cancer in women with an elevated risk
Study | MRI sensitivity | Mammography sensitivity |
|---|---|---|
Kreige et al. [4] | 71.1 % | 40 |
N = 1,909 | ||
Warner et al. [7] | 77.3 | 36.4 |
Leach et al. [6] | 77 | 40 |
N = 649 | ||
Saridenelli et al. [8] | 93.8 | 58.8 |
N = 278 | ||
Weinstein et al. [9] | 71 | 39 |
N = 609 | ||
Lehman et al. [12] | 100 | 33.3 |
N = 171 | ||
Morris et al. [11] | 100 | |
N = 365 | ||
Podo et al. [10] | 100 | 12.5 % |
N = 105 |
The role of MRI as a supplement to mammography and whole breast ultrasound has been reported by Berg and others. The supplemental yield of additional cancers was 14.7 per 1,000 women screened using breast MRI. Among women screened with MRI, 2.6 % were diagnosed with breast cancer [15]. The sensitivity and value of MRI has been therefore clearly proven in these studies. The number of screens needed to detect one cancer was 127 for mammography, 234 for supplemental breast ultrasound, and 68 for MRI after a negative mammogram and ultrasound. The sensitivity and PPV3 [positive predictive value] for combined mammography and ultrasound were 44 and 18 %; for combined MRI, mammography, and ultrasound, they were 100 and 19 % [15]. Among the 612 women who had MRI in addition to mammography and ultrasound, the rate of biopsy increased from 6.2 to 13.2 % because of the addition of MRI. The PPV3 for MRI was 19 % [15]. The increased cancer detection rate varied between 1.2 and 6.7 % and was accompanied by a positive predictive value ranging from 23.7 to 60 %. MRI will lead to increased biopsies; the reported range is from 4.6 to 16.1 % [1].
Role of Breast MRI in a Patient with Known Breast Cancer
Indications for use of breast MRI in a patient diagnosed with breast cancer for staging and to assess response to chemotherapy are discussed in detail in Chap. 9.
Breast MRI to Assess Integrity of Implants
There are many different types of breast implants that complicate imaging assessment. About 14 types have been described [16]. Approximately 80 % of the implants are placed for cosmetic reasons and about 20 % are placed as a part of reconstructive surgery. A large majority of breast implants are single-lumen silicone gel implants, about 80 % in a series of nearly 10,000 implants. Saline-filled, dextran-filled, and PVP-filled implants have similar appearances on MR imaging. An understanding of the various types of implants and their component features improves accuracy in assessment of these implants [16]. There are three common types of implants: the single-lumen silicone gel, which consists of an outer silicone capsule containing viscous silicone gel, the single-lumen inflatable saline implant with greater chances of deflation when ruptured, and the double-lumen implants. The latter are of two types, one in which the inner lumen is filled with silicone and the other in which there is a saline-filled inner lumen and silicone-filled outer lumen [17].
Gel leaking or leeching refers to microscopic leakage of silicone through semipermeable membrane leading to capsule formation and contracture [17]. To minimize the chances of this happening decreasing the gel concentration and placement of silicone barriers on the inner surface of the envelope has been tried. Implants may be placed subglandular or subpectoral. The incidence of capsular contraction is higher with the subglandular implants and is likely due to direct contact of the implant with breast tissue. MR imaging is performed to identify implant rupture and has been shown to be the most reliable modality to diagnose implant-related complications [16–23]. The diagnosis of an implant rupture is important because the release of silicone gel and fluid into tissues can lead to local complications [18]. The incidence of implant rupture is 1–2 %; the rate of silent rupture is considerably higher [16]. Rupture may be suspected due to symptoms such as tenderness, palpable nodules, asymmetry, or infection. Implant rupture may be asymptomatic and be discovered during clinical examination, particularly when the rupture is intracapsular, where free silicone remains inside the fibrous capsule that develops around the implant. In an extracapsular rupture, free silicone is seen in the breast tissue outside the implant. Mammography is of limited use in the assessment of implant rupture and is able to diagnose extracapsular rupture only in which case free silicone is seen in the breast parenchyma [17]. Ultrasound is more useful in the assessment of breast implants but is less accurate than is MRI. Diffuse low-level echoes when seen is suggestive of an implant rupture [19]. A contour abnormality is an unreliable sign of implant rupture [19]. Common implant-related complications include hematoma in the early postoperative period, infection, capsule contracture, rupture, and formation of silicone granulomas [19]. More recent studies have reaffirmed the accuracy of MR imaging in assessment of implants [22, 23]. An accuracy of 90–92 %, sensitivity of 89–96 %, specificity of 77–97 %, positive predictive value of 90–99 %, and a negative predictive value of 79–90 % have been reported [22, 23]. The linguine sign and the salad oil signs were statistically the most significant signs [23]. Presence of silicone granulomas, free silicone, and silicone in axillary lymph nodes are suggested as signs that require immediate explantation (Table 8.2) [23]:
Table 8.2
MR signs of breast implant rupture
Definite sign of implant rupture | Possible rupture | Not indicative of a rupture |
|---|---|---|
Linguine sign | Teardrop sign | Irregular margin |
Subcapsular line | Noose sign, keyhole sign | Lobulated contour |
Train sign | Droplets in silicone | Simple radial folds |
Salad oil sign | Complex radial folds | |
Extracapsular silicone |
Linguine Sign/Subcapsular Line: These two signs are the most reliable signs of an implant rupture and appear as hypointense lines that are wavy and appear folded within the silicone gel and lie parallel to the fibrous capsule; these represent the ruptured silicone shell floating within the fibrous capsule (Fig. 8.1a, b). Subcapsular line is a prelude to the linguine sign when the detachment from the fibrous capsule is not complete [22].
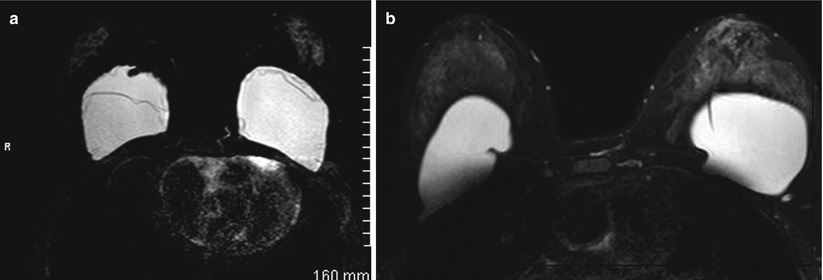
Fig. 8.1
(a) A silicone-excited sequence shows bilateral implant rupture with typical “linguine sign” within the implants representing collapsed implant shell. (b) A silicone-excited sequence shows normal infolding of the implant shell simulating a “linguine sign”
Teardrop Sign, Noose Sign, or Keyhole Sign: These signs are a result of invagination of the silicone membrane containing a drop of silicone; hence, the membranes are not opposed or touching each other as in folds. This finding depending on the shape may appear as a teardrop, noose, or a keyhole. When such an appearance is seen in more than one image, it is suggestive of an implant rupture.
Droplet Sign or the Salad Oil Sign: Dot-like hypointensity within silicone represents the presence of water or serum droplets within the silicone gel. By itself this sign cannot be considered diagnostic of an implant rupture and may even represent a normal finding if saline steroids or antibiotics are directly injected into the silicone chamber in the perioperative period [23]. When larger this sign is called as the salad oil sign, a finding that is diagnostic of an implant rupture [23].
Train Rail Sign: Two hypointense parallel lines are seen in close proximity forming a double-contoured subcapsular line within the silicone gel indicating that both membranes in a double-lumen implant have ruptured [22].
Simple and Complex Radial Folds: Radial folds represent normal infolding of the implant shell and may simulate the linguine sign of implant rupture; these are normal findings that represent uninterrupted hypointense lines, and these extend almost perpendicularly into the lumen and end blindly (Fig. 8.1b). Complex folds are longer and have a multidirectional course. Use of orthogonal planes and reduced slice thickness or volumetric acquisitions help. Patient motion artifacts can also sometimes cause curvilinear hypointense lines within the implant simulating the linguine sign [22, 23].
Contour Change/Irregular Margin: Contour changes and irregular margin when by itself is not a sign of implant rupture and may indicate herniation of the fibrous capsule. Rupture without collapse has been attributed to some cases of implant rupture that was missed on MR imaging [20]. The ruptured surface elastomer in these cases adhered to the fibrous capsule without producing the linguine sign. The homogenous high signal was maintained within the ruptured implant.
Extracapsular Silicone: This is a sign of extracapsular rupture of an implant. There is free silicone in the soft tissue or a silicone granuloma which may appear as dense rounded or irregular mass.
Breast MRI as a Problem-Solving Tool
MR imaging is not recommended for routine use as a problem-solving tool to supplement diagnostic mammography. There have been studies that have examined the value of MRI as a supplemental modality for equivocal findings on mammography [24–27]. MRI is not recommended for lesion characterization or biopsy avoidance. However, in routine practice occasional use of breast MRI is made to further assess suspected abnormal findings. Judicious use of MRI is important due to the cost and potential for false positives. The ACR Practice Guideline for the Performance of Contrast-Enhanced MRI of the breast includes “additional evaluation of clinical or imaging findings” as one of the indications for performing breast MRI. The guideline specifically states that “breast MRI may be indicated when other imaging examinations, such as ultrasound and mammography, and physical examination are inconclusive for the presence of breast cancer, and biopsy could not be performed.” The guideline goes on to caution, however, that “MRI should not supplant careful problem-solving mammographic views or ultrasound in the diagnostic setting” and “should not be used in lieu of biopsy of a mammographically, clinically, and sonographically suspicious finding” [28].
In one series problem solving was an indication for 3.9 % of MRI exams of the breast performed over a 6-year period; there were 115 exams performed for inconclusive findings that represented 0.14 % of the total number of mammograms performed [24]. The most common indication was focal asymmetry [85 %] followed by architectural distortion [10 %] and scar at site of benign breast biopsy [4 %]. A majority of cases were classified as BI-RADS 0 prior to MRI [68 %]; in 19 % an assignment of BI-RADS 4 was made and MRI was performed as a biopsy avoidance tool. Ultrasound was performed in 65 of these 115 cases with no malignancies found. MRI of the breast identified six malignancies and had a sensitivity of 100 %; two of these six cancers were seen on one mammographic view. The positive predictive value of MRI was 14 % [24]. The role of MRI in downgrading BI-RADS 3 lesions has been reported but is not a cost-effective approach for this indication. The negative predictive value of MRI in reported studies for noncalcified BI-RADS 3 lesions was 100 %. For probably benign calcifications, the negative predictive value was 76–97 % for BI-RADS 3 microcalcifications [25]. For this reason there is no justification for use of MRI downgrading BI-RADS microcalcifications. A report on use of MRI as an adjunct to mammography found that MRI had the most benefit in lesions that were characterized as BI-RADS 0 or 3. A significant higher sensitivity was achieved with the use of MRI with nearly similar specificity [26]. Cost-effectiveness remains an issue despite the beneficial findings shown in these few studies and was not addressed. MRI should generally not be used for lesion characterization or for biopsy avoidance since percutaneous biopsy under imaging guidance is relatively safe, less expensive, and readily available [1]. Moreover, for this indication to be valid, MRI has to have a greater than 98 % negative predictive value which has not been the case. A large series of 821 patients with a suspicious mammographic or clinical finding found an NPV of only 85 % with cancer missed in 48 of 329 negative MR examinations [27]. Based on lack of robust data and issue of cost-effectiveness, it seems prudent to use MRI occasionally as a problem-solving tool in cases such as lesions seen on one view and sonographically occult [13].
Breast MRI to Diagnose an Occult Breast Cancer
Uncommonly, adenocarcinoma is identified in axillary lymph nodes with no mammographic evidence of a primary in the breast. Such a presentation is seen in less than 1 % of breast cancer cases [1]. Such metastasis is usually from the ipsilateral breast. Identifying a tumor may result in less radical surgical procedures and/or radiation depending on tumor size, characteristics, and extent [1]. MRI successfully identifies occult primary cancer in 61 % of cases [1]. The European Society of Breast Imaging recommends use of MRI in case of localized metastatic disease such as axillary adenopathy when clinical and conventional imaging fail to identify a breast primary [5]. When metastasis is extensive and prognosis is poor and will not be affected by site of primary tumor, there is no role for the use of breast MRI [5].
MRI BI-RADS Lexicon
Dynamic contrast-enhanced MR mammography is now an accepted modality for screening, diagnosing, and staging of breast cancer. With increasing utilization of breast MRI, there was a need to standardize terminology, reporting, and final assessments to fall in line with what had already been established and used for mammography and breast ultrasound. The American College of Radiology incorporated the BI-RADS™ MRI lexicon into its Breast Imaging and Data System Atlas in 2003 [29]. An updated version is nearly complete and due to be released later this year with significant changes made to the original lexicon [30].
The descriptors are for types of enhancement, location of the lesion, the kinetic time-intensity information, and associated findings [31, 32]. There are two main categories of descriptors of enhancing lesions, namely, the morphology and the enhancement kinetics. There are three morphologic types of enhancing lesions that will be discussed next.
Focus or Foci
Focus or foci are enhancing lesions that are small and typically less than 5 mm; these are often related to hormonal changes and are benign (Fig. 8.2a, b). The finding of a focus or foci is often stable on follow-up examination. Foci may be challenging to assess for enhancement kinetics due to volume averaging effect. The differential diagnosis of such foci includes focal fibrocystic change, small fibroadenoma, papilloma, benign lymph node, or rarely DCIS or a small invasive ductal cancer [32]. In a retrospective study of 666 MR-only detected lesions that underwent histological confirmation, the incidence of cancer among foci was less than 3 %; for this reason biopsy is rarely needed for foci particularly when there are more than one such finding [33].
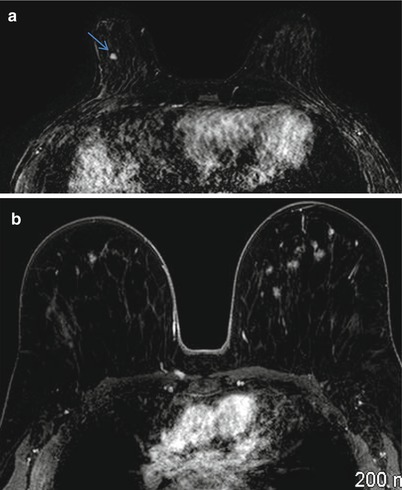

Fig. 8.2
(a) Axial postcontrast fat-suppressed T1-weighted subtraction image shows a 4 mm enhancing focus in the right breast. (b) Axial postcontrast fat-suppressed T1-weighted subtraction image shows multiple bilateral enhancing foci
Masses
A mass is larger than 5 mm and is three dimensional and visible on precontrast images. This may indicate an invasive breast cancer or a benign entity such as a fibroadenoma. The shape, margins, and internal enhancement characteristics are described next.
Shape of a Mass
This may be described as round, oval, or lobulated when the border is undulating. The shape is considered irregular when it has an uneven shape and cannot be categorized as round, oval, or lobulated. A round mass is circular or ball shaped, an oval mass is elliptical, a lobulated mass can have a scalloped contour.
Margins of a Mass
This feature can be described as being smooth, irregular, or spiculated. The latter is suspicious for cancer or a radial scar. Smooth margin is well defined with sharp demarcation from surrounding tissue (Fig. 8.3a–c). An irregular margin is uneven, ill-defined, or indistinct and can have jagged edges. A spiculated margin has spicules radiating from the surface. An irregular mass or one with an irregular or spiculated margin is commonly associated with invasive breast cancer.
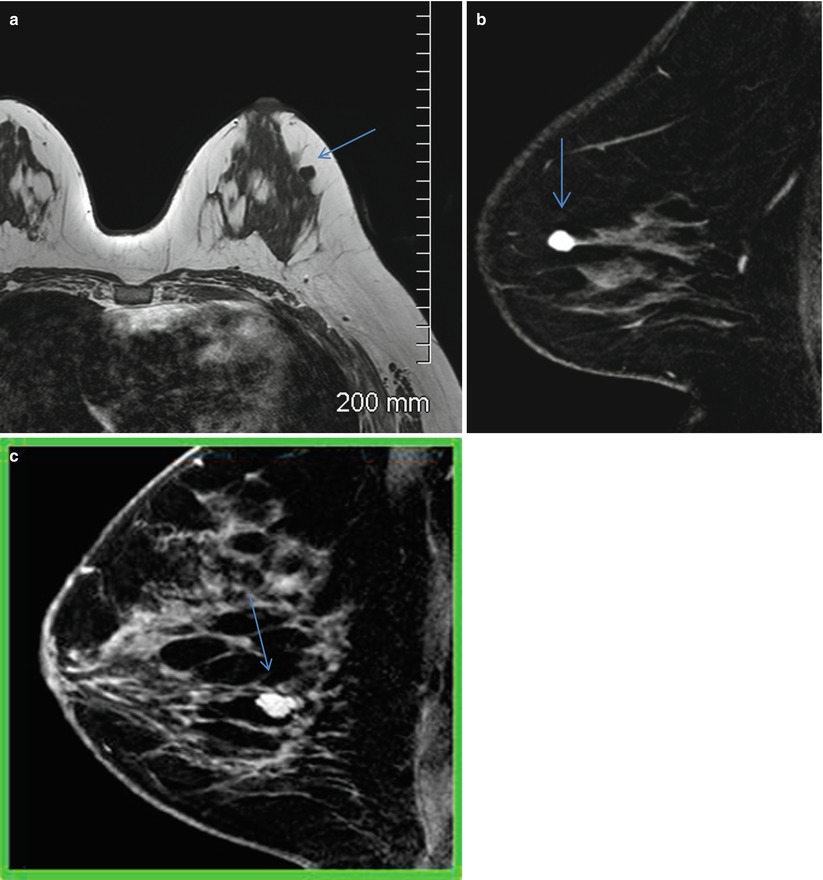

Fig. 8.3




(a) Axial T1-weighted image shows an isointense mass with smooth borders in the outer central left breast. (b) Sagittal postcontrast fat-suppressed T1-weighted image shows homogenous enhancement and smooth margins suggestive of a benign mass. Histological diagnosis: fibroadenoma. (c) Sagittal postcontrast fat-suppressed T1-weighted image shows enhancing mass with dark internal septations and smooth margins suggestive of a benign mass. Histological diagnosis: fibroadenoma
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree