THE CHANGING INCIDENCE OF MALIGNANCIES IN PATIENTS WITH HUMAN IMMUNODEFICIENCY VIRUS DISEASE
The relationship between malignancies and acquired immunodeficiency syndrome (AIDS) changed in 1996 when highly active antiretroviral therapy (HAART) was introduced in industrial nations. Thanks to the United Nations and other philanthropy programs, HAART has also been successfully introduced into a number of developing nations (1). Africa, the pandemic epicenter, is the exception, due to the epidemic magnitude on that continent and its significant political and social turmoil. Prior to 1996, epidemiologists noted specific malignancies afflicting patients with AIDS, with a risk proportional to host immune status. Before HAART, AIDS patients could be separated into two groups: patients with an opportunistic infection as their first manifestation of AIDS (60%) and those with a malignancy as its mode of presentation (40%) (2).
Of those with an AIDS-related malignancy, up to 90% would have Kaposi sarcoma (KS) and the rest non-Hodgkin lymphomas (NHLs), including primary central nervous system lymphoma (PCNSL) and systemic diffuse large B-cell lymphoma (DLBCL). Despite an increase in human papilloma virus (HPV)-related invasive cervical cancers in women with high-grade uterine cervical dysplasia, recent findings of a lack of clear association between cervical cancer and human immunodeficiency virus (HIV)-related immunosuppression questions the validity of including cervical cancer among AIDS-defining or associated malignancies (3). After HAART, previously obvious relationships between AIDS and some malignancies have been challenged. An example is HIV-related Burkitt lymphoma, initially associated with AIDS-induced immunosuppression. Investigators have found that HAART improvement of immunity is associated with significant reductions in KS, PCNSL, and systemic DLBCL, but this is not the case with Burkitt lymphoma. Together with invasive cervical cancer, the incidence of Burkitt lymphoma has remained stable across the pre- and post-HAART eras, increasing its proportional frequency. Epithelial dysplasia and squamous cell carcinomas of the anal canal, rectum, and oral cavity are also observed in men infected by HIV. There has been a post-HAART era increase in Hodgkin lymphoma (Epstein-Barr virus related), lung cancer, and nonmelanoma skin cancer, with implications related to the complex relationship between immunity, aging, chronic antigenic stimulation, and viral oncogenesis. Overall, the excess risk of a malignancy in HIV disease is observed mostly in cancers with an established or suspected infectious cause (4).
In this chapter, we first discuss HIV and its effect on the immune system. We then concentrate on malignancies associated with AIDS immunosuppression. We include a discussion of malignancies (Burkitt lymphoma and HPV-related cancers) not directly associated with HIV immunosuppression occurring with a high enough incidence in HIV-infected patients to merit study.
HUMAN IMMUNODEFICIENCY VIRUS DISEASE
The AIDS pandemic came into the medical world in 1980 with the publication in the Center for Diseases Control and Prevention (CDC) journal Morbidity and Mortality Weekly Report of a series of patients afflicted by de novo opportunistic infections, mostly Pneumocystis jiroveci (formerly known as Pneumocystis carinii) pneumonia and cytomegalovirus (CMV) infections (5). At about that time, these conditions were known to occur in immunodeficient patients. It was followed by a report of KS in 26 homosexual men thought to be immunologically healthy. The number of similar cases increased exponentially throughout the United States and Europe, with reports from other parts of the world confirming a new pandemic with an epicenter in sub-Saharan Africa. The high number of cases with similar clinical presentations gave way to a new syndrome, the acquired immunodeficiency syndrome, or AIDS. Twenty-five years after, much is known and much is to be learned about the cause of the syndrome, HIV, and its complications, including opportunistic infections and malignancies (6).
Human immunodeficiency virus disease is caused by infection of a human subject with a retrovirus, HIV. Human immunodeficiency virus is only transmitted through blood or unprotected sexual contact, causing a progressive destruction of the immune system with occurrence of opportunistic infections and malignancies. When an opportunistic infection or a malignancy occurs, it signals a significant degree of immunodeficiency. The association of opportunistic infections and immune suppression is well established in the medical literature and clinical practice. The relationship between immune surveillance dysfunction and development of malignancies has also been described in the medical literature. In the early 1970s, kidney transplantation programs in Canada reported an increased incidence of malignancies in patients treated with the immunosuppressant azathioprine (7). Similar reports indicated that pharmacologically immune-suppressed patients had increases of several folds of magnitude of certain types of cancers and opportunistic infections. Typically, they had KS or lymphoproliferative malignancies, although other types of malignancies were also reported. In a sense, these patients had a chemically induced AIDS, whereas HIV patients have a biologically induced AIDS.
Human immunodeficiency virus was discovered in 1983 (8), dispelling much of the mystery surrounding AIDS. Acquired immunodeficiency syndrome (AIDS) in humans is caused by a retrovirus, HIV, a lentivirus (Latin lentus, meaning “slow,” + virus), endemic in African primates, which entered the human population as a result of a cross-species transmission or zoonosis. Two types of HIV have infected humans: HIV-1 and HIV-2. The HIV-1 virus originated from chimpanzees infected by a retrovirus, the SIVcpz (the chimpanzee simian immunodeficiency virus), and HIV-2 originated from sooty mangabeys monkeys, endemically infected by another retrovirus, SIVsm. SIVcpz is the product of recombination in chimpanzees of two monkeys’ retroviruses preyed upon by chimpanzees: the SIVrcm and the SIVgsn (the red capped mangabeys, Cerocebus torquatus, and the great spot-nosed monkeys, Ceropithecus nictitans, respectively). These viruses do not cause disease in their natural hosts and were named simian immunodeficiency viruses, or SIV, after their genetic and structural similarities with HIV. Each HIV type (1 and 2) is phylogenetically classified in groups and clades; HIV-1 has three groups, M (major), N (non-M, non-O), and O (outlier), with the predominant group M comprising 11 clades A to K, and HIV-2 has six clades, A to F. The HIV-1 and HIV-2 clades can recombine giving origin to genetically complex viral quasispecies. Transmission from animals to humans occurs when infected animals enter in contact with humans as a result of hunting and butchering (SIVcpz from chimpanzees) or through contact with infected animals used as domestic pets (sooty mangabeys monkeys) (9).
The HIV-2 virus is endemic and contained in coastal West Africa. The HIV-1 virus is endemic in west equatorial Africa with several clades from the group M responsible for the majority of worldwide infections. In the Western world, HIV-1B is responsible for over 90% of infections, whereas in Africa, subtypes A, C, D, and G, and in Asia, subtype C and the circulating recombinant forms (CRFs) 01 and 02 can be found. The disease is diagnosed by detecting serum anti-HIV antibodies using an enzyme-linked immunoabsorbent assay (ELISA) test. The initial result can be corroborated by a repeated Elisa test (Explanation: saying “with another Elisa test” implies a different type of ELISA) or by a Western blot blood test. The amount of replicating virus can be measured by quantitative polymerase chain reaction (PCR) or via signal amplification such as branch-DNA HIV viral load test (10).
Human immunodeficiency virus disease and AIDS are responsible for a human tragedy of incalculable proportions. Since the pandemic’s beginning, there have been over 25 million deaths and more than 50 million infected persons, including those who have died. The estimated rate of new infections is 7,500 new infections per day. Most of these new infections occur in developing nations, affecting many women and children. The most frequent modes of transmission are unprotected sex and intravenous (IV) drugs. The main epicenter of the pandemic continues to be sub-Saharan Africa, with new epicenters in the former Soviet Union, China, India, and Latin America. In the United States, minority groups such as Hispanics and African Americans as well as younger generations of gay men are disproportionately affected by HIV (11).
Human immunodeficiency virus is an enveloped diploid RNA virus (two RNA ribbons per particle), each with an armamentarium of enzymes essential for the virus life cycle. The viral particle membrane is composed of human leukocyte antigen (HLA) groups, other cell surface membrane proteins, and the viral protein gp120 in a trimer form. Among the enzymes within the viral particle are reverse transcriptase, integrase, and proteases. Eighty percent of transmitted mucosal HIV infections are established by a single Transmitted/Founder (T/F) variant, or Transmitted/Founder virus. The other 20% are transmitted by two to five variants (ie, IV drug users). These T/F viruses have specific phenotypic characteristics, including enhanced infectivity, higher envelope content, more efficient binding of dendritic cells, and relative resistance to interferon (IFN)-α (12). Once HIV enters a susceptible host, the viral envelope spikes (gp120 trimers) bind to receptors (CD4) and co-receptors (CCR-5) of cells of the immune system, entering through a membrane fusion. The particle content is released into the cytoplasm, and viral RNA is transcribed into DNA. This viral DNA is then randomly integrated into the genome of the infected cell. Through a complex process of transcription activators and as result of the frequent replication cycles of the immune system’s cells, new viral particles are generated. These new particles are released through budding and lysis, continuing to infect new susceptible cells with this process, occurring at an unusually fast pace. Infection with HIV is characterized in simian models of AIDS and in man by a rapid destruction of memory cells of the gut-associated lymphoid tissue (GALT). This process occurs in days to few weeks in animal models and in weeks in man. GALT harbors the majority of body lymphocytes in comparison to the 2% to 5% of lymphocytes located in the peripheral circulation.
The result of GALT’s destruction, together with the activation of B cells and inhibition of the immune system function by HIV viral gene products such as the vif protein, which inhibits the APOBEC3 gene family, implicated in the control of HIV infection including the production of neutralizing antibodies, inducing a severe immune dysfunction and depletion. (This is an important piece of information that address the mechanistic question of why we have such a difficult time developing neutralizing antibodies against HIV) (13). Once HIV is integrated into the genome of the susceptible cells, there is a gradual destruction of the immune system, leading to a progressive status of immunodeficiency, with development of opportunistic infections and tumors. This early senescence of the immune system is implicated in changes at a molecular level resulting in loss of control of oncogenic viruses and associated with the malignant transformations observed in AIDS.
ACQUIRED IMMUNODEFICIENCY SYNDROME–DEFINING MALIGNANCIES
Kaposi sarcoma was described by Dr. Moritz Kaposi in 1872 as an indolent dermatologic disease characterized by the appearance of purplish nodules or plaques, particularly in the lower extremities of older men of Eastern European, Mediterranean, or Jewish descent (classical KS). Canadian investigators were one of the first ones to note in the 1970s that KS occurred in renal transplant patients exposed to immunosuppressant regimens (ie, azathioprine; transplant or iatrogenic KS). Their observations included reports of remissions when immunosuppressant regimens were temporarily discontinued, suggesting a relationship between immunodeficiency and the malignancies. They also noted a high incidence of NHL. In the 1960s, British investigators reported an aggressive lymphadenopathic form of KS confined to equatorial Africa (African KS). This form occurred in younger patients with aggressive involvement of lymph nodes and appearance of nodules and plaques of the lower extremities that rapidly became ulcerated. With the CDC Morbidity and Mortality Weekly Report of 26 gay men with KS, the disease became one of the hallmarks of the AIDS epidemic (epidemic or AIDS-related KS).
In contrast to the classical form, the AIDS-related KS lesions appear with an aggressive pattern of distribution that includes the trunk, arms, and face in addition to the lower extremities (14). Prior to antiretroviral therapies, patients died of a combination of tumor progression and opportunistic infections. Despite HAART, KS continues to be a prevalent cancer among HIV-infected patients, although HAART has significantly changed the incidence of the disease. From 1990 to 1995, the incidence of KS in the United States was 1,838.9 cases per 100,000 person-years in contrast to 334.6 cases per 100,000 person-years from 1996 to 2002. In the United States and Europe, AIDS-related KS has been almost exclusively diagnosed in homosexual men, suggesting that its prevalence may vary among different categories of AIDS patients. In Africa, where the human herpesvirus 8 (HHV-8), or KS herpesvirus (KSHV), is endemic, the male-to-female ratio of AIDS-related KS in some countries is 2:1, almost the same ratio observed in transplant- or iatrogenic-related KS. Thus, in the presence of profound immune suppression, factors that made the disease more prevalent in males than in females prior to the AIDS pandemic appear to be of little relevance. What is clear is that the incidence of AIDS-related KS is related to the degree of the immune suppression of the infected hosts, with most afflicted patients having CD4+ cell counts of 200 CD4+ cells/μL or less.
The etiologic agent of all forms of KS is HHV-8, also called KSHV (15). Early in the pandemic, other viruses or agents were implicated as the cause of AIDS-related KS, including CMV. In 1994, sequences of a new herpes-like virus were isolated from the lesions of an AIDS-related KS patient. Using a subtractive PCR technique called representative differential analysis, investigators found sequences isolated from KS lesions homologous but not identical to other known herpesviruses, and thus it was named HHV-8, because it became the eighth known herpesvirus. Not all patients infected with HHV-8 develop KS; however, viral DNA and seroconversion can be detected in patients prior to the development of KS, confirming the role of HHV-8 as the cause of all forms of KS and the relationship of its pathogenesis to immunosuppression in addition to other cofactors.
Human herpes virus 8 belongs to the γ-herpesvirus subfamily and the subgroup γ-2 or rhadinovirus (from the Latin term rhadino, referring to the tendency of the viral genome to break apart when it is isolated) and is the first human virus of this subfamily identified. The detection of the infection relies on the presence of antibodies against viral antigens using immunofluorescence assays based on the use of B lymphocytes as the antigen source or ELISA with recombinant immunogenic proteins or peptides of HHV-8. The infection seroprevalence mirrors the geographic distribution of AIDS-related KS, with the highest infection rates in central African countries (80%), rates of 25% to 50% among homosexual men in the Western world, and an intermediate level in the Mediterranean regions. The adult general population of blood donors in North America and Europe has an HHV-8 seroprevalence ranging from 0% to 8%. In addition to being the etiologic agent of AIDS-related KS, HHV-8 has been associated with two other lymphoproliferative disorders: primary effusion lymphoma (PEL, a subset of body cavity–based lymphomas [Fig. 44-1], subsequently called PELs) and multicentric Castleman disease (15).
Human herpes virus 8 incorporates a significant number of host genes such as cyclin D and growth factor interleukin (IL)-6 (16). These genes participate in the replication, survival, and transformation of the infected tumor cells. The viral K1 gene kaposin and viral G protein–coupled receptor (vGPCR) have transformation potential. Others deregulate cell growth and lead to transformation including viral IL-6, viral IL-10, viral cc-class chemokines, and viral FLICE-inhibitory protein (vFLIP). The expression of the different key genes is related to the latency and lytic cycles of HHV-8. During the latency phase, genes such as LANA-1 (latency-associated nuclear antigen 1), in addition to the maintenance of latency, inactivate p53, inhibiting apoptosis. In addition, a viral cyclin prevents cell cycle arrest by cyclin-dependent kinases, pRB and vFLIP, avoiding the activation of the Fas death receptor pathway. During the lytic phase, homologues of replication genes including the K1 kaposin gene, a Bcl-2 homologue, a viral G protein–coupled receptor gene (vGPCRP), a viral homologue of IL (IL-6), and viral macrophages and IFN regulatory factors become active. Some of these genes have immunosuppression functions, such as the inhibition by vFLIP including cytotoxicity of T cells against HHV-8–infected cells and the inhibition of HHV-8 class 2 major histocompatibility complex (MHC)-mediated T-cell activation by K1 (17). Finally, other viral proteins such as K3 and K5 downregulate the presentation of MHC class 1 molecules on the cell surface.
The histology of KS is characterized by the abundance of spindle cells in a matrix of neovascular formation and a rich background of mononuclear inflammatory cells and collagen. Vascular spaces are dilated and contain extravasated erythrocytes. Involvement of the reticular dermis, reflected by patchy lesions and the involvement of all the layers of the skin, clinically presents as nodular or plaque lesions that can coalesce, interfering with the lymphatic circulation, and are histologically and clinically associated with surrounding hemorrhage and subcutaneous edema. The spindle KS cells are rich in endothelial factor VIII. Recent microarray studies have demonstrated that the origin of the KS cell is from a virally transformed lymphatic endothelial cell. Kaposi sarcoma spindle cells express angiogenic/inflammatory cytokines and growth factors, including vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), IL-1, and IL-6, among others. Kaposi sarcoma cells also overexpress receptors for cytokines, suggesting growth through autocrine or paracrine mechanisms. They also proliferate in response to IL-1, IFN-γ, IL-6, and tumor necrosis factor (TNF), which are abundantly present in the serum of patients with poorly controlled HIV infection, and matrix metalloproteinases (MMPs), enzymes involved in the destruction of extracellular matrix proteins required for angiogenesis and metastasis. In AIDS-related KS, Tat (the trans-activator of transcription protein) stimulates KS spindle and endothelial cell replication, promoting an increase in the concentrations of bFGF. This, in turn, upregulates the integrins α5β1 and αvβ3, receptors for fibronectin and vitronectin, which are highly expressed in AIDS-related KS.
Most patients with AIDS-related KS have CD4+ cell counts of 200 CD4+ cells/μL of blood, with an increased number and aggressiveness of lesions in those who are more severely immunosuppressed. There are periods of exacerbation alternated with quiescence related to oscillations of the patient’s immunity. Kaposi sarcoma rarely invades the central nervous system. This is of biological interest because many other human malignancies are characterized by the invasion of the central nervous system (18).
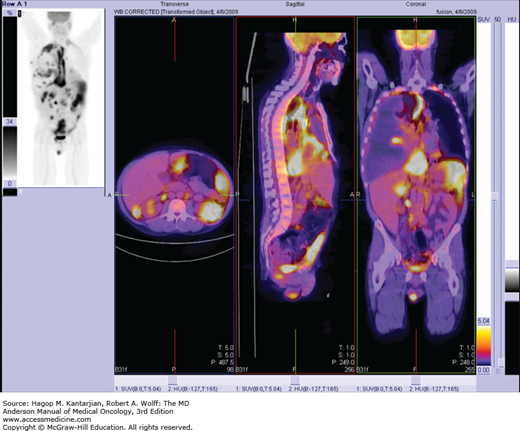
The distribution of skin lesions in patients with AIDS-related KS often follows the Langer’s folds of the skin. The occurrence of lesions in acral regions of the body such as the tip of the nose is common. The evolution of the skin lesions correlates with the patient’s immune system status. In the pre-HAART era, severe involvement of the skin of the face by raised purplish lesions was frequent. With KS progression, there is frequent involvement of the gastrointestinal tract. Lesions of the palate and gums are often the first ones to be noted, and diarrhea and occasional bleeding can suggest KS involvement of the gastrointestinal tract. In the case of involvement of the lower extremities, progressive edema with nodular and coalescing plaque lesions can cause significant discomfort and pain. Prior to HAART, this was a frequent and serious complication of KS, because “elephantiasis” secondary to the progression of KS was extremely difficult to treat. Advanced cases often involve ulceration of lesions, particularly when located in the lower extremities. Lymph node involvement is frequent, and when there is only generalized lymph node involvement, a biopsy is required for confirmation of diagnosis. In advanced cases, lung involvement is manifested by bilateral basilar infiltrates mixed with a nodular appearance; however, severe hemoptysis or gastrointestinal bleeding is infrequent. During the early years of the epidemic, a significant number of patients experienced involvement by large masses of KS in vital organs such as the liver and heart, and death was due to from progression of their KS tumors and associated opportunistic infections.
The usual TNM system used in other solid tumors is not easily applicable to AIDS-related KS. A system based on the work of Chachoua and colleagues was proposed in 1989 by the AIDS Clinical Trial Group (ACTG) (Table 44-1). This staging system included the extent of tumor involvement, the immune status measured by the level of CD4+ cell count, and presence or absence of any systemic illness (B symptoms). In addition to a complete physical examination, it included a complete blood count, serum chemistries, HIV viral load, panendoscopy of the gastrointestinal tract, computed tomography (CT) of the abdomen and pelvis, and when indicated, the performance of bronchoscopy when pulmonary involvement by KS was suspected. Biopsies of skin or lymph nodes were also suggested when indicated to rule out entities that could be similar in presentation, such as bacillary angiomatosis or pyoderma gangrenosum. After HAART, the extent of disease and the presence of HIV systemic symptoms became the most important prognostic factors; however, pulmonary involvement by KS still carries a particularly poor prognosis. Correlations with the levels of HIV viral load and the status of HHV-8 infection are under study in relationship to their impact on survival (19).
| Characteristics | Good Risk (0) | Poor Risk (1) |
|---|---|---|
| All of the Following | Any of the Following | |
| Tumor (T) | Tumor confined to skin and lymph nodes and/or minimal oral diseasea | Tumor-associated edema or ulceration; extensive oral KS; gastrointestinal KS; KS in other nonnodal viscera |
| Immune system (I) | CD4 cells ≥150/mm3 | CD4 cells <150/mm3 |
| Systemic illness (S) | No history of opportunistic infection or thrush; no B symptomsb; performance status ≥70 (Karnofsky) | History of opportunistic infection and/or thrush; B symptoms; performance status <70 (Karnofsky); other HIV-related illness (eg, neurologic disease, lymphoma) |
Highly active antiretroviral therapy brought a dramatic decrease in the incidence of AIDS-related KS. Highly active antiretroviral therapy consists of the administration of a combination of agents with anti-HIV activity, including inhibitors of HIV reverse transcriptase and protease inhibitors. The consensus of experts in the field about frontline components of HAART is periodically published in Guidelines for the Use of Antiretroviral Agents in HIV-1 Infected Adults and Adolescents by the Department of Human and Health Services (DHHS; available online by visiting the DHHS website). For patients in whom KS is part of the initial diagnosis of AIDS, HAART therapy should be started irrespective of the extent of the disease. For patients with minimal tumor burden, HAART initiation constitutes frontline therapy of their AIDS-related KS. This approach can control these lesions for long periods of time, often more than 1 year, and in many instances results in complete disappearance of KS lesions (20). Patients with extensive disease or visceral involvement can receive systemic chemotherapy in addition to HAART.
Radiotherapy can be useful for treatment of minimal local disease and when the use of systemic treatment other than HAART is not indicated. It can also be used as an adjunct treatment modality for patients in whom the administration of chemotherapy leads to incomplete results, enhancing the beneficial effects of the systemic treatment. Depending on the general condition of the patient and the size of the lesions to be treated, doses range from the administration of a single fraction to fractionated doses over periods of 2 to 4 weeks. For single lesions and frail patients, the administration of a single 800-cGy dose can be used. Radiotherapy can be used for cosmetic reasons, although this should be done carefully to avoid secondary side effects such as postradiation cataracts in the case of periorbital lesions. For larger lesions or when the therapeutic intent is cosmetic, fractionated doses between 200 and 4,000 cGy are effective and carry less risk. For patients receiving systemic therapy, radiotherapy can be an adjuvant for the treatment of complicated single lesions, particularly when they are bleeding, ulcerated, or painful, or when they affect the well-being of the patient. Such is the case of patients with disseminated disease receiving systemic treatment and in whom oral lesions may affect eating due to local pain or size.
In the modern era, the use of local therapies such as cryotherapy and laser therapy may have a role for patients with few and small lesions. The use of surgery may be appropriate in selected cases such as large skin lesions or when there are complications (bleeding of obstruction of a hollow viscus). Other treatments, such as intralesional injection of chemotherapeutic agents, particularly of the oral cavity, and application of alitretinoin gel, have been abandoned and replaced by a more sophisticated use of radiotherapy techniques, HAART, and systemic chemotherapy.
After the demonstration of the activity of IFN-α in hairy cell leukemia and renal cell cancer in 1984 (21), there was an impetus to use the same doses of IFN in patients with AIDS-related KS. Low doses of IFN-α effective against hairy cell leukemia and renal cell carcinoma were ineffective against AIDS-related KS (Rios A, personal observation). A dose-response study unequivocally demonstrated the therapeutic effect of IFN-α in KS when used at doses of 20 to 30 MU/m2 (22,23). A different situation was observed with IFN-γ. Under the angiogenic stimuli of IFN-γ, KS has the capacity to replicate, resulting in a deleterious impact on patients treated with this agent in pilot studies. As a result of these trials, recombinant IFNs α-2a (Roferon-A) and α-2b (Intron-A) were approved for the systemic treatment of patients with AIDS-related KS. Expanded use of these agents has revealed their true activity to be in the range of 15% to 20%.
Interferon can block the synthesis of viral proteins and the budding of viral particles from infected cells in addition to other complex pleiotropic effects. Interferon actions are accompanied by significant systemic side effects including tiredness, fatigue, anorexia, hepatotoxicity, and severe myelosuppression. With the development of HAART and the use of more effective systemic chemotherapy regimens in the treatment of AIDS-related KS, the interest in the use of IFN-α in the treatment of AIDS-related KS has declined.
Indications for the use of systemic chemotherapy in AIDS-related KS includes extensive skin, mucocutaneous, and visceral involvement by tumor. In patients who require systemic chemotherapy, local radiotherapy is used to treat local complications in addition to systemic disease. The introduction of HAART has resulted in better and more durable responses with increased tolerability and durability than those observed prior to HAART.
Before the discovery of antiretrovirals, a variety of chemotherapeutic agents had modest to significant activity as monotherapy for KS. These agents included etoposide, vinblastine, vincristine, bleomycin, doxorubicin, vinorelbine, and epirubicin, which induced responses in 40% to 69% of patients. After HAART, ABV (doxorubicin 20 mg/m2, bleomycin 10 U/m2, and vincristine at maximum doses of 1 to 2 mg) became the first standard treatment of AIDS-related KS. It produced a response rate of 60%, with complications and tolerance depending on the performance status and general condition of the patient (23). Antiretroviral and other supportive therapies with growth factors (granulocyte-macrophage colony-stimulating factor [CSF] and granulocyte CSF [G-CSF]) paired with vigorous prophylaxis of opportunistic infections reduce the risks of treatment. Complications of ABV were the expected ones with systemic chemotherapy including the potential for cardiac toxicity induced by doxorubicin.
The ABV regimen was followed by the introduction of agents considered today the standard of care for AIDS-related KS, including liposomal encapsulated anthracyclines (doxorubicin and daunorubicin) and taxanes (paclitaxel). The last of these promotes apoptosis and downregulates Bcl-2 protein expression in KS cells in vitro and in KS-like lesions in mice. In addition, it has an important antimitotic effect associated with its capacity for the disruption of tubulin activity during mitosis.
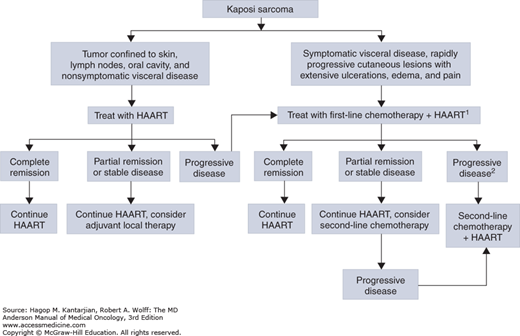
The current treatment of AIDS-related KS is based on the combination of an anthracycline (liposomal doxorubicin 20 mg/m2 or liposomal daunorubicin 40 mg/m2 but not both together) with paclitaxel 25 mg/m2 with or without bleomycin or vincristine. Escalation of the dose of liposomal doxorubicin is not recommended due to a syndrome of desquamation of the skin of the palms and soles of the feet, known as palmar-plantar erythrodysesthesia. In contrast, the dose of liposomal-encapsulated daunorubicin can be increased to up to 60 mg/m2 or even higher for patients who tolerate lower doses. This is of particular relevance in patients with advanced disease or significant pulmonary involvement and for whom prompt control and achievement of a quick therapeutic response is of great importance (Fig. 44-2).
FIGURE 44-2
Algorithm for the management of acquired immunodeficiency syndrome (AIDS)-related Kaposi sarcoma. 1Monthly evaluation of Kaposi sarcoma clinical response and estimation of CD4+ cell count and HIV-RNA levels. 2Highly active antiretroviral therapy (HAART) regimen should be changed in the case of immunovirologic failure. (Reproduced, with permission, from Catellan AM, Trevenzoli M, Aversa SM. Recent advances in the treatment of AIDS-related Kaposi sarcoma. Am J Clin Dermatol. 2002;3:451-462.)
Only patients with early disease and relatively good performance status consistently achieve durable remissions with current therapies for KS. For the rest of the patients, only palliation and stabilization of disease is achieved with current treatments. For these reasons, efforts are under way to develop new therapies based on the knowledge of the pathophysiology of the disease. For example, because angiogenesis is an important component of AIDS-related KS, agents such as thalidomide and anti-VEGF agents such as bevacizumab are of great interest in the therapy of this disease. Metalloprotease inhibitors are also of great interest, and active clinical trials are in progress. Viruses associated with the production of malignancies tend to constitutively activate the nuclear factor-κB (NF-κB) pathway, and agents that can inhibit this pathway such as bortezomib may be of some value. Inhibition of signaling cell receptors implicated in the stimulation of angiogenesis such as platelet-derived growth factor receptor (PDGFR) and C-kit receptor by agents such as imatinib, an orally administered tyrosine kinase inhibitor, approved by the US Food and Drug Administration (FDA) for treatment of chronic myeloid leukemia and gastrointestinal stromal tumor, is being investigated.
There is significant interest in the development of therapies against the latent phase of HHV-8, the most common form of HHV-8 in KS cells, which does not respond to standard antiherpetic drugs such as foscarnet and cidofovir. This area of research has led to potential development of a vaccine against HHV-8. Despite all these new potential therapeutic developments, the impact of HAART in the incidence of AIDS-related KS cannot be overemphasized. The development of more potent and less toxic HAART regimens and the acceptance of earlier therapeutic intervention against HIV seem to be the main paths to control the epidemic of AIDS-related KS.
Non-Hodgkin lymphoma is the second most frequent AIDS-associated malignancy. Both KS and NHL were occurring with an incidence almost linear in its relationship to the patient’s immunodeficiency status (24). In 1985, high- or intermediate-grade B-cell NHLs were considered part of the spectrum of AIDS-related malignancies. Eighty percent of AIDS-related NHLs were systemic (peripheral) lymphomas, involving nodal or extranodal sites, with 15% to 20% originating in the primary central nervous system (PCNSL). A small proportion, less than 3%, of systemic AIDS-related NHL patients had PELs, known as body cavity lymphomas. In general, the risk of AIDS-related NHL in patients with HIV appears to be higher in those who have poor immune function with average CD4+ cell counts of 150 CD4+ cells/μL of blood.
A viral relationship is implicated in the development of AIDS-associated lymphomas. Epstein-Barr virus (EBV) contributes to the development of most of these tumors, although HHV-8 is associated with the development of PEL (24). There is no relationship between the risk of development of AIDS-related NHL and modes of HIV transmission. The incidence of pre-HAART AIDS-related NHL was 60 to 200 times higher than in a matched HIV-seronegative population; the relative risk was higher for PCNSL. Age, nadir of CD4+ cell count, and absence of anti-HIV therapy were critical factors that predicted the development of AIDS-related NHL. In the pre-HAART era, 80% of these NHLs, including systemic and PCNSL cases, were immunoblastic variants associated with CD4+ cell count depletion and EBV infection. In the post-HAART era, there has been a 30% reduction of peripheral cases and a 70% reduction in PCNSL, indicating the impact of immune reconstitution in the incidence of immunosuppression-related lymphomas. In contrast, the incidence of Burkitt lymphoma and of centroblastic DLBCL has remained stable without significant change from the pre- to the post-HAART eras (25). When comparing the AIDS-related lymphomas with non–AIDS-related NHL, the former tend to be of higher histologic grade, with increased frequency of B symptoms, extranodal presentations, and an increased incidence of leptomeningeal and primary CNS involvement (26). In the post-HAART era, the World Health Organization (WHO) has expanded the categories of lymphomas that can occur in HIV patients to include extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue type (MALT lymphoma), peripheral T-cell lymphoma (PTCL), and classical Hodgkin lymphoma (HL), as well as lymphomas that more specifically occur in AIDS patients, including plasmablastic lymphomas of the oral cavity, polymorphic B-cell lymphomas (posttransplant lymphoproliferative disorder–like), and PEL (27). Finally, the demographics of AIDS-related NHL patients have changed in the last decade, reflecting changes in the demographics of the AIDS epidemic, with increasing incidence in Hispanic and African American patients and patients who have acquired HIV through heterosexual contact (Table 44-2).