PANCREATIC CANCER
When clinicians use the term pancreatic cancer, they refer to adenocarcinoma of the pancreas, one of the most challenging malignancies facing oncologists today. This disease is characterized by significant morbidity and poor prognosis.
At the University of Texas MD Anderson Cancer Center (MDACC), we manage patients with pancreatic cancer with a multidisciplinary team and view palliation as the primary goal. However, for patients with potentially resectable disease, we take an aggressive approach whenever appropriate. In the setting of advanced disease, cure is not possible, but as our understanding of carcinogenesis, invasion, and metastasis expands, more effective therapeutic strategies are expected to emerge. This chapter reviews our current knowledge about pancreatic cancer, including its epidemiology, risk factors, molecular biology, diagnosis and staging, and clinical strategies for therapy.
HARD FACTS ABOUT PANCREATIC CANCER
Pancreatic cancer, the most common pancreatic neoplasm, is an aggressive and often rapidly fatal malignancy. In the United States, it represents 2% of all cancer cases but accounts for 5% of all cancer deaths (1). Currently, it is the fourth leading cause of cancer death, ranking behind lung, colorectal, and breast cancer. While evidence suggests marginal improvements in 5-year survival rates over the last 25 years (2% in 1974-1976, 3% in 1983-1985, and 4% in 1992-1997), life expectancy remains short and is generally measured in months (2). By 2030, deaths due to pancreas cancer are projected to increase dramatically and will become the second leading cause of cancer-related death (3). Significant improvements in survival have been hampered by a number of factors, including inefficient screening strategies, technically challenging and often debilitating surgery, and minimally effective chemotherapy and radiotherapy.
Pancreatic cancer is a dynamic disease, and sudden changes in clinical status occur frequently. Patients may rapidly develop worsening pain, biliary obstruction, or stent occlusion with cholangitis, thromboembolism, peritoneal carcinomatosis, or intractable ascites. Any of these problems may preclude the timely delivery of cytotoxic therapy and limit survival. Therefore, most efforts should focus on symptom control, but for patients with adequate performance status (PS), treatment is encouraged.
EPIDEMIOLOGY
There are approximately 45,000 new cases of pancreatic cancer each year in the United States and 330,000 cases worldwide. Incidence rates are highest in industrialized societies and Western countries. Of note, the risk of pancreatic cancer among African Americans, in whom pancreatic cancer mortality rates are higher than most other ethnic groups in the United States, is considerably higher than the rates for African blacks (4). These observations implicate environmental factors conspiring with genetic background as causes of the increased risk.
The risk of developing pancreatic cancer is low in the first three to four decades of life but increases sharply after the age of 50. Average age at the time of diagnosis is 72 years. Pancreatic cancer is uncommon in patients under 40. In the past, pancreatic cancer occurred more frequently in men, but now the disease is becoming more common in women, probably secondary to the increased use of tobacco by women.
RISK FACTORS FOR PANCREATIC CANCER
Surprisingly, relatively little is known about the risk factors for the development of pancreatic cancer. Table 21-1 summarizes genetic and environmental factors associated with an increased risk.
| Acquired Risk Factors | Relative Risk | Comments |
|---|---|---|
| Tobacco smoking | 2-5 | Risk increases with increasing exposure |
| Diabetes mellitus | 2 | Not all authorities concur; many patients have altered glucose metabolism on presentation |
| High body mass index | 2 | |
| Chronic pancreatitis | 13-18 | Not all authorities concur with this degree of increased risk |
| Inherited Disorders | Relative Risk | Known Defects |
| Hereditary pancreatitis | 10-53 | PRSS1 |
| FAMMM syndrome | 22 | p16INK/CDKN2 |
| HNPCC | 8 | MLH1, MSH2, MSH6 |
| Peutz-Jeghers syndrome | 13-30 | LBK1/STK11 |
| Familial adenomatous polyposis | 4-5 | APC |
| Li-Fraumeni syndrome | ? | p53 |
| Familial breast and ovarian cancer | 3-5 | DNA repair pathways, BRCA2 |
Aside from age, the only consistently reported risk factor for pancreatic cancer is cigarette smoking. Cigarette smoking is estimated to account for roughly 30% of pancreatic cancer mortality (5). Experimental models have demonstrated that nitrosamines found in tobacco smoke are carcinogenic for the pancreas. Research performed at MDACC has shown that smoking-related aromatic DNA adducts and other types of DNA damage may be critical in carcinogenesis.
Diabetes has been implicated both as an early manifestation of pancreatic cancer and as a predisposing factor. A meta-analysis published between 1975 and 1994 showed that pancreatic cancer occurred with increased frequency in patients with long-standing diabetes (diagnosed at least 5 years prior to the diagnosis or death due to pancreatic cancer) (6). It is believed that insulin resistance and secondary hyperinsulinism may be involved in pancreatic carcinogenesis.
Early clinical studies have suggested an association between chronic pancreatitis and pancreatic cancer. In a study of 715 patients with chronic pancreatitis diagnosed between 1971 and 1995, there was a 13- to 18-fold increase in pancreatic cancer rates (7).
Studies have suggested that hereditary pancreatitis, in particular, may affect the risk. Lowenfels and Maisonneuve studied 246 patients with hereditary pancreatitis from 10 countries. The estimated cumulative risk of pancreatic cancer by age 70 was approximately 40%, with a mean age at diagnosis of 57 years (8). Molecular data strongly suggested that mutations in the trypsinogen gene PRSS1 play an important role in hereditary and possibly acquired forms of pancreatitis (9).
Positive associations have been discovered between pancreatic cancer and meat and carbohydrate intake. However, there is no consensus on the contribution of dietary fat. Epidemiologic studies of pancreatic cancer have shown a protective role for high fruit and vegetable intake. This effect may be related to dietary folate and other methyl donor groups.
Epidemiologic studies have also implicated a high body mass index as increasing risk for pancreatic cancer. This may be explained by relative hyperinsulinemia thought to promote pancreatic carcinogenesis (10).
Patients with pancreatic cancer who have two first-degree relatives with a history of pancreatic cancer are defined as having familial pancreatic cancer. The estimated relative risk for other family members is increased 10- to 20-fold over the general population. In addition, hereditary nonpolyposis colorectal cancer, ataxia-telangiectasia, Peutz-Jeghers syndrome, familial breast and ovarian cancer, and familial atypical multiple-mole melanoma are all associated with increased risk of pancreatic cancer (11).
The familial breast and ovarian cancer syndrome, associated with mutations in the BRCA genes, accounts for 17% of cases (12). The BRCA1 and BRCA2 proteins are key components of homologous recombination through the repair of DNA double-strand breaks. Cells deficient in these proteins demonstrate genomic instability and a tendency toward malignant transformation (13). The poly(ADP-ribose) polymerase (PARP) enzyme is integral for single-stranded DNA repair, and inhibition results in DNA breaks that require intact BRCA proteins for repair. In the presence of mutant BRCA proteins, PARP inhibition results in synthetic lethality (14).
Preclinical work has demonstrated the sensitivity of BRCA mutated pancreatic cancer cell lines to cross-linking chemotherapeutic agents, such as mitomycin-c and cisplatin (15). This finding has translated clinically as studies have shown superior overall survival and response in patients with advanced pancreatic cancer with BRCA mutations treated with platinum (13). Furthermore, studies with PARP inhibitors have shown promise either alone or in combination with cytotoxic chemotherapy (16). Currently, an ongoing study is investigating the efficacy of a single-agent PARP inhibitor, rucaparib, in patients with advanced pancreatic cancer with known BRCA mutations.
Exposure to carcinogens in the workplace has been implicated in pancreatic cancer, but with the possible exception of formaldehyde, the available evidence is insufficient to identify any specific exposure to increase the risk.
Surgical procedures such as gastrectomy and cholecystectomy have been reported to increase risk and are possibly linked to elevated levels of cholecystokinin and hypergastrinemia. However, other studies have not demonstrated such an association (17).
MOLECULAR EVENTS IN HUMAN PANCREATIC CARCINOGENESIS
The molecular events leading to pancreatic cancer have not been fully elucidated, but mutations in a few oncogenes and tumor suppressors appear critical for carcinogenesis. Investigators from Johns Hopkins performed a comprehensive genomic analysis of pancreatic cancer reported in the journal Science. They noted an average of 63 alterations, the majority of which were point mutations. These alterations defined a core set of 12 cellular signaling pathways and processes that were altered in the majority of pancreatic cancers. These included the K-ras, wnt/notch, hedgehog, TGF-β, integrin, and JNK signaling pathways (18). Recently, Waddell et al characterized pancreatic cancer into four subtypes termed stable, locally rearranged, scattered, and unstable (19).
Pancreatic cancer has the highest frequency of K-ras mutation among all human cancers. Greater than 85% of cases have an activating point mutation in the K-ras gene, most commonly a G-to-T transversion at codon 12 (20). This leads to constitutive activation of RAS, leading to growth-promoting signal cascades via the mitogen-activated protein (MAP) kinase pathway. Preclinical models have implicated this mutation as a very early event in carcinogenesis. The ras oncogene mutations were also retrospectively seen in pancreatic juice collected 3.5 years prior to a patient’s diagnosis of pancreatic cancer (21).
The p16 tumor suppressor gene is inactivated in approximately 95% of pancreatic cancers; this typically occurs later in carcinogenesis. The second most frequently inactivated tumor suppressor gene, p53, located on chromosome 17p, also appears to be a late event in tumorigenesis. The DPC4 gene (SMAD4) is inactivated in 55% of pancreatic adenocarcinomas and, like p53, is a relatively late event in tumorigenesis. In a comprehensive mutational analysis of 42 pancreatic ductal cancers, Rozenblum and colleagues found individual mutational frequencies of tumor suppressor genes p16, p53, DPC4, and BRCA2 were 82%, 76%, 53%, and 10%, respectively (21).
Current data suggest a temporal sequence of molecular events in pancreatic carcinogenesis leading from an “adenomatous” or proliferative ductal cell phenotype to pancreatic intraepithelial neoplasia (Pan-IN) and finally to invasive cancer. This theory has been supported by the recognition of noninvasive proliferative ductal lesions (Pan-IN1) that demonstrate mutations commonly found in pancreatic cancer. Growing evidence suggests that the gradual accumulation of genetic and biochemical alterations in early lesions causes progression to higher levels of dysplasia (Pan-IN2 and Pan-IN3) and ultimately to cancer.
The overexpression of epidermal growth factor receptor (EGFR), vascular endothelial growth factor (VEGF), matrix metalloproteinases, cyclooxygenase 2 (COX-2), hedgehog signaling, and insulinlike growth factor type 1 (IGF-1) pathways have been implicated in pancreatic cancer. Studies conducted at MDACC have also demonstrated that the nuclear transcription factor–κB (NF-κB) is commonly activated in pancreatic cancer (22).
Hedgehog signaling is an essential pathway during embryogenesis of the normal pancreas, and its dysregulation has been reported in precancerous PanIN-1 and -2 as well as in primary and metastatic pancreatic adenocarcinomas. Inhibition of hedgehog signaling by cyclopamine-induced apoptosis and blocked proliferation in vitro and in vivo (23). Although debatable, hedgehog inhibition also depletes tumor-associated stroma and may play a role in the delivery of chemotherapeutic agents like gemcitabine (24). Clinical trials of hedgehog inhibitors have failed to create a meaningful impact in this disease. Recent data indicated that stromal depletion may actually be deleterious, suggesting a critical need for accurate interpretation of preclinical data before incorporation into the clinical trial setting (25).
Insulinlike growth factor type 1 upregulates cell proliferation and invasiveness through activation of the phosphoinositide 3-kinase (PI3K)/Akt signaling pathway and downregulates the tumor suppressor chromosome 10 (PTEN). Akt mediates the gemcitabine and erlotinib resistance mechanisms. At MDACC, we recently concluded a clinical trial with dalotuzumab (MK-0646), which targets the IGF-1 pathway.
Targeted therapy has been a major focus of clinical research, taking priority over the study of more conventional cytotoxic agents. Preliminary studies with agents designed to abrogate RAS function have been disappointing (26). Likewise, administration of inhibitors of matrix metalloproteinases has failed to demonstrate a meaningful clinical impact (27). Recent studies of EGFR, VEGF, NF-kB, or COX-2 inhibitors are discussed in further detail in this chapter. Immune therapies may have a significant impact as well.
PATHOLOGY
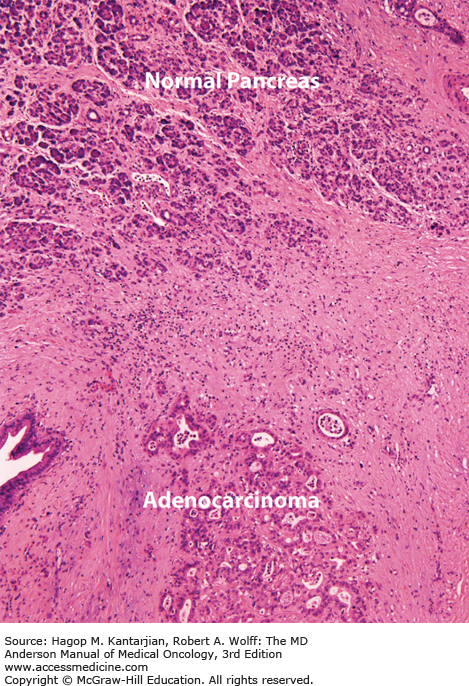
Pancreatic acinar cells account for approximately 80% of the cell number and volume of the gland, with islet cells accounting for 1% to 2%. The ductal system is made up of single-layer, cuboidal epithelial cells comprising 10% to 15% of the gland’s structure, with a sparse interlacing network of blood vessels, lymphatics, nerves, and collagenous stroma. In carcinoma, this architecture is markedly altered: The predominant histologic feature is a dense collagenous stroma with atrophic acini, remarkably preserved islet cell clusters, and a slight-to-moderate increase in the number of normal-appearing and cancerous ducts (Fig. 21-1). The diagnosis of ductal adenocarcinoma rests on the identification of mitoses, nuclear and cellular pleomorphism, discontinuity of ductal epithelium, and evidence of perineural, vascular, or lymphatic invasion.
Almost all malignant neoplasms of pancreatic origin (95%) arise from the exocrine portion of the gland. Tumors arising from the islets of Langerhans (endocrine) cells are much more infrequent, and primary nonepithelial tumors (eg, lymphomas or sarcomas) are extremely rare. The histologic classification of exocrine pancreatic neoplasms is presented in Table 21-2.
| Malignant |
| Ductal adenocarcinoma |
| Mucinous cystadenocarcinoma |
| Acinar carcinoma |
| Unclassified large cell carcinoma |
| Small cell carcinoma |
| Pancreatoblastoma |
| Benign |
| Serous cystadenoma |
| Variable malignant potential |
| Intraductal papillary mucinous tumor |
| Mucinous cystadenoma |
| Papillary cystic neoplasm |
CLINICAL PRESENTATION
The clinical presentation of pancreatic cancer is primarily dependent on the location of the tumor within the pancreas. The majority (85%) develop within the pancreatic head. About 10% are located in the pancreatic body and 5% in the tail. Nonspecific, poorly localized, epigastric or back pain is the most common initial presentation. It is usually caused by invasion or compression of the celiac, splanchnic, or mesenteric plexi. Tumors in the head or neck typically cause pain in the epigastric area or in the right upper quadrant of the abdomen. Cancers of the body may cause unremitting, severe back pain, and tumors in the pancreatic tail are associated with left upper quadrant pain.
Painless jaundice, another common presentation, is generally associated with tumors in the pancreatic head or uncinate process. When the tumor does not arise in proximity to the intrapancreatic portion of the bile duct, diagnosis may be delayed and characterized by abdominal pain or back pain without jaundice.
Acute pancreatitis, while uncommon, can be caused by a ductal adenocarcinoma; in patients with no other reason for acute pancreatitis (lack of gallstones, no history of alcohol or precipitating drugs) (28). Symptoms of chronic pancreatitis are relatively common, including diarrhea, bloating or constipation, abdominal distention, and weight loss. Patients with tail lesions often present with signs or symptoms of metastatic disease.
DIAGNOSIS AND STAGING OF PANCREATIC CANCER
Pancreatic cancer can be difficult to diagnose, particularly in patients with nonspecific complaints. Therefore, patients who present to the oncologist for treatment recommendations may harbor feelings of frustration and anger, having endured a significant delay from the onset of symptoms to diagnosis. Upper endoscopy may have been performed to rule out peptic ulcer disease or other pathology. Endoscopy is seldom helpful unless the pancreatic tumor has invaded the adjacent gastric or duodenal mucosa, leading to ulceration. In this uncommon situation, biopsies may demonstrate adenocarcinoma, and subsequent cross-sectional body imaging reveals an underlying pancreatic mass. Even more rarely, extrinsic compression on the gastric or duodenal wall may be appreciated endoscopically. Unfortunately, upper endoscopy may be misleading and demonstrate mild esophagitis, gastritis, or duodenitis, with or without evidence of Helicobacter pylori. Alternatively, patients complaining of right upper quadrant pain may undergo ultrasonography, potentially revealing gallstones, prompting cholecystectomy. This procedure is usually of temporary benefit and delays the discovery of a pancreatic tumor until pain returns. Last, for patients presenting with complaints of back pain, a musculoskeletal evaluation commonly ensues, with the procurement of plain x-rays, myelograms, or magnetic resonance imaging (MRI) of the spine.
For those patients who present with obstructive jaundice, suspicion of pancreatic cancer is sufficiently high that the diagnostic workup usually proceeds in an orderly fashion with directed imaging studies. These usually include an abdominal ultrasound, computed tomographic (CT) scan of the abdomen, or both. In some centers, discovery of a mass in the head of the pancreas without obvious metastatic disease or evidence for unresectability will prompt an exploratory laparotomy prior to biopsy confirmation of malignancy. This approach is not embraced at MDACC for reasons outlined further in this chapter.
With rare exception, all patients seen at MDACC are advised to undergo tissue confirmation. Cross-sectional imaging (multidetector CT) should always be performed before interventional endoscopic or radiologic procedures to prevent procedure-related inflammatory changes from confounding assessment. For patients presenting with obstructive jaundice, tissue may be obtained at the time of endoscopic retrograde cholangiopancreatography (ERCP) via brushings of the bile duct at the level of stricture. If brushings are nondiagnostic and CT or MRI suggests that the tumor may be nonmetastatic, we advise endoscopic ultrasound (EUS) with EUS-guided fine-needle aspiration (FNA) (29). This can be performed by experienced operators with minimal risk of duodenal perforation. Moreover, it is thought to decrease the risk of peritoneal or needle-track seeding, which has been reported among patients undergoing transcutaneous ultrasound- or CT-directed biopsies (30). Alternatively, when CT or MRI clearly demonstrates an unresectable, locally advanced cancer, CT- or ultrasound-guided transcutaneous biopsy may substitute for EUS-guided aspiration. If a patient presents with obstructive jaundice and biliary stricture without radiographic evidence of a pancreatic mass, EUS examination is also advised.
When there is radiographic evidence of metastatic disease and an obvious pancreatic mass, we recommend biopsy of a metastatic site, such as the liver. This confirms both the diagnosis and the presence of metastatic disease with one procedure.
It is not uncommon for patients to be misdiagnosed with pancreatic cancer. The most common mistake we see is in the setting of bulky peripancreatic adenopathy without a parenchymal pancreatic mass. Adenocarcinomas of the pancreas do metastasize to regional lymph nodes, but lymph nodes are typically small to medium in size. Bulky lymph nodes are seen in other gastrointestinal (GI) malignancies, such as tumors of the esophagus, stomach, duodenum, and occasionally, colon. Lymphoma, non–small cell lung cancer, and carcinomas of unknown primary origin may also lead to bulky peripancreatic lymphadenopathy, thus mimicking a primary pancreatic neoplasm. Thin-cut, dynamic-phase, contrast-enhanced CT will usually rule out the presence of a primary mass in the pancreas in this setting. Another helpful radiographic finding may be the presence or absence of atrophy of the pancreatic body and tail. Although commonly seen in adenocarcinomas originating in the head of the pancreas, this finding is usually absent in the setting of bulky peripancreatic adenopathy, neuroendocrine tumors, and acinar cell tumors. Importantly, patients with neuroendocrine tumors of the pancreas are sometimes misdiagnosed as having a poorly differentiated carcinoma of the pancreas.
The single most important imaging modality is multidetector (multislice) CT. This technique is used to objectively define (anatomically) potentially resectable disease. For optimal pretreatment staging, a CT report in a patient with suspected pancreatic cancer should include the following:
The presence or absence of a primary tumor in the pancreas or periampullary region
The presence or absence of peritoneal and hepatic metastases
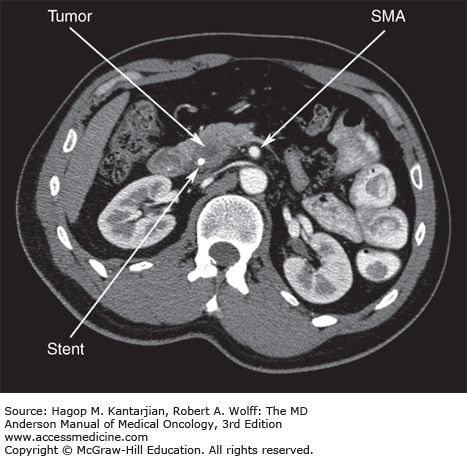
Description of the patency of the superior mesenteric vein (SMV) and portal vein (PV) and the relationship of these veins to the tumor
Description of the relationship of the tumor to the superior mesenteric artery (SMA), celiac axis, and hepatic artery
Objective radiographic criteria can be used to define a potentially resectable primary tumor of the pancreatic head or uncinate process (Fig. 21-2). The MDACC criteria include: (1) no extrapancreatic disease; (2) a patent SMV and PV (assuming the technical ability to resect and reconstruct this venous confluence); and (3) a definable tissue plane between the tumor and regional arterial structures, including the celiac axis and SMA. Using CT staging and objective criteria for assessment of resectability, many centers have reported resectability rates as high as 75% to 80% (31). Of note, CT of the chest is not routinely part of our staging workup. However, if either plain chest x-rays or CT images of the lung bases reveal pulmonary nodules or other suspicious findings, a dedicated CT scan of the chest is obtained. Bone scans and brain imaging are rarely indicated and should not be part of routine staging.
Positron emission tomographic (PET) scans are occasionally obtained in the setting of equivocal radiographic findings, such as indeterminate lesions in the liver or lungs. Lesions may be subcentimeter in size, and 18F-fluorodeoxyglucose negative, even when metastatic disease is present (32). Scanning by PET is commonly considered when a patient has undergone previous resection and subsequently develops a rising CA19-9 and soft tissue changes in the surgical bed with no other evidence of relapse.
CA19-9 measures the specific carbohydrate moiety of the mucin MUC-1 (33). This is the most commonly elevated tumor marker in pancreatic cancer, but it is not specific and may be elevated in other GI tumors. Whether it should be measured prior to surgery as an independent predictor of resectability or as an adjunct to other clinical staging has not been rigorously studied. Most retrospective analyses generally suggested that a high preoperative CA19-9 level (>500-1,000 IU/mL) implies more advanced disease that is not amenable to resection. We retrospectively analyzed pretreatment CA19-9 levels obtained from 79 patients enrolled in a trial of gemcitabine-based preoperative chemoradiation. All patients had radiographically defined, biopsy-proven, resectable cancer without evidence of metastatic disease. It was found that serum levels greater than 668 IU/mL predicted either the development of overt metastatic disease prior to surgery or early relapse after surgical resection (34). Presently, serum CA19-9 measurements are obtained at presentation to MDACC. When postoperative CA19-9 levels do not normalize within this time frame, it portends early relapse (35). Patients clinically staged as having locally advanced disease but with markedly elevated CA19-9 levels (>5,000) are suspected of having occult metastatic disease. These patients are usually advised to undergo a trial of systemic therapy with serial measurements of CA19-9 levels prior to considering chemoradiation. Improvement of CA19-9 of 50% has been correlated with an improved survival (36).
For patients with potentially resectable disease, some have advocated laparoscopy with biopsies and peritoneal washings as part of routine staging (37,38). In our experience, fewer than 20% of patients with tumors in the head of the pancreas will have occult metastatic disease when laparoscopy is performed. Given the expense and expected negative findings for 80% of patients, our approach has been to limit this procedure to patients with indeterminate findings on CT (39). An exception applies to the small subset of patients who present with radiographically resectable tumors in the body or tail of the pancreas who are more likely to have occult metastatic disease. The chance of a visible peritoneal metastasis or positive cytology on peritoneal washing is sufficiently high to justify laparoscopy as part of staging (40).
The TNM (tumor, node, metastasis) staging system for pancreatic cancer is outlined in Table 21-3. For patients undergoing resection, the TNM system is somewhat useful in providing prognostic information and, to a lesser degree, in guiding adjuvant therapy. Generally, patients are staged as having potentially resectable disease, locally advanced unresectable disease, or metastatic disease. For patients with resectable disease who are able to tolerate it, surgery is indicated. Surgery can be preceded by preoperative or adjuvant therapy. Patients with metastatic disease and adequate PS usually receive systemic therapy. For patients presenting with locally advanced disease, treatment should be individualized and may initially involve either chemoradiation or systemic therapy.
| Tx: | Primary tumor cannot be assessed | ||
| T0: | No evidence of primary tumor | ||
| Tis | Carcinoma in situ | ||
| T1: | Tumor <2 cm in greatest dimension | ||
| T2: | Tumor >2 cm in greatest dimension | ||
| T3: | Tumor extends beyond the pancreas but without involvement of the celiac axis or the superior mesenteric artery | ||
| T4: | Tumor involves the celiac axis or the superior mesenteric artery (unresectable primary tumor) | ||
| Nx: | Regional lymph node status cannot be assessed | ||
| N0: | No regional lymph node metastasis | ||
| N1: | Positive regional lymph node metastasis | ||
| Mx: | Distant metastasis cannot be assessed | ||
| M0: | No distant metastasis | ||
| M1: | Distant metastasis | ||
| Staging Classification | |||
| Stage | T | N | M |
| 0 | Tis | N0 | M0 |
| IA | T1 | N0 | M0 |
| IB | T2 | N0 | M0 |
| IIA | T3 | N0 | M0 |
| IIB | T1-T3 | N1 | M0 |
| III | T4 | Any N | M0 |
| IV | Any T | Any N | M1 |
TREATMENT STRATEGIES FOR PANCREATIC CANCER
It is widely known that surgery holds the only hope of cure for patients with pancreatic cancer. With some exceptions, resectable pancreatic cancers are limited to small tumors in the head of the pancreas. These are removed with a Whipple procedure (41), more appropriately described as a pancreaticoduodenectomy. Caution is advised when considering a resection of a tail neoplasm; even when these tumors appear localized, they are associated with a higher likelihood of peritoneal seeding compared to a head lesion. Body lesions are almost never amenable to resection.
Unfortunately, there are potential drawbacks associated with up-front surgery:
Surgical morbidity and mortality are inversely correlated to experience with the procedure. Several studies have confirmed significant differences in the risk of major perioperative complications and death between hospitals that perform the operation frequently and those that do not (42,43). Moreover, long-term survival after pancreaticoduodenectomy is improved when performed at a high-volume center (44). This is likely attributable to a combination of decreased operative mortality and superior patient selection.
Positive surgical margins are associated with a very poor prognosis (see Table 21-4). Surgical margins after pancreaticoduodenectomy can be either microscopically positive (R1 resection) or grossly positive (R2). Median survivals with a positive surgical margin usually range between 6 and 12 months, similar to, if not worse than, the survival of patients with locally advanced disease (45,46). Many patients undergo laparotomy without adequate preoperative assessment. Some will be found to have unresectable tumors intraoperatively or be left with a grossly positive surgical margin when it might have been possible to predict this prior to surgery. Patients may therefore have a delay in chemoradiation or systemic therapy while the patient recovers. With the exception of patients who present with gastric outlet obstruction or biliary obstruction not amenable to endoscopic or percutaneous stenting, we discourage surgical intervention without high-quality radiographic evidence of resectability. If the surgeon has failed to perform a complete resection, surgery may even be deleterious and compromise the patient’s survival.
A substantial proportion of patients do not recover sufficiently to receive postoperative adjuvant therapy. Pancreaticoduodenectomy is a major surgical procedure with removal of portions of the stomach, duodenum, pancreas, and bile duct requiring extensive reconstruction of the upper alimentary canal. Pancreatic anastomotic leaks and delayed gastric emptying are common complications. Retrospective analyses and prospective clinical trials of adjuvant therapy demonstrated that a significant percentage of people do not adequately recover to receive postoperative therapy. For example, Johns Hopkins University demonstrated that of 870 patients who underwent resection for pancreatic cancer with curative intent between 1993 and 2005, only 53% received adjuvant therapy (47). Furthermore, analyses of Medicare-eligible patients suggested that among patients 65 years of age or older, fewer than half receive adjuvant therapy (48). It is reasonable to assume that a substantial proportion of elderly patients have sufficient difficulty recovering from surgery and this has an impact on adjuvant therapy.
Surgically resected patients remain at risk for local failure and metastatic disease. Approximately 80% of resected patients will ultimately relapse and die of disease recurrence. The high risk of relapse stems from an inability to prevent locoregional failure and to eradicate microscopic metastatic disease. Factors predisposing to local recurrence have not been fully elucidated, but recent evidence implicated perineural invasion as an important mediating process. Invasion of nerve sheaths may occur as a pervasive superficial infiltration that cannot be appreciated intraoperatively, even by the most experienced surgeons.
| Author | Number of Patients | Margin Status | Median Survival (Months) |
|---|---|---|---|
| Sohn | 184 | R1/R2 | 12 |
| Neoptolemos | 101 | R1 | 11 |
| Nishimura | 70 | R1/R2 | 6 |
| Millikan | 22 | R1 | 8 |
| Richter | 72 | R1/R2 | 12 |
| Kuhlman | 80 | R1/R2 | 16 |