BASIC CONCEPTS
High-dose chemotherapy (HDC) with autologous hematopoietic progenitor cell (HPC) transplant is an effective treatment modality for several hematologic malignancies and selected solid tumors. This chapter reviews its current role in the treatment of cancer and outlines promising future directions of progress.
High-dose radiation and chemotherapy are limited by toxicity to normal tissues, particularly the bone marrow. The doses of certain chemotherapeutic agents and radiation can be substantially escalated, with the goal of exploiting their dose-response effect, when followed by autologous or allogeneic transplantation of HPCs to restore hematopoiesis. Pluripotent HPC progenitors present in the graft proliferate and differentiate into the mature blood and immune cells. Autologous transplantation involves collection, cryopreservation, and infusion of the patient’s own HPCs.
GENERAL PROCEDURES FOR AUTOLOGOUS TRANSPLANTATION
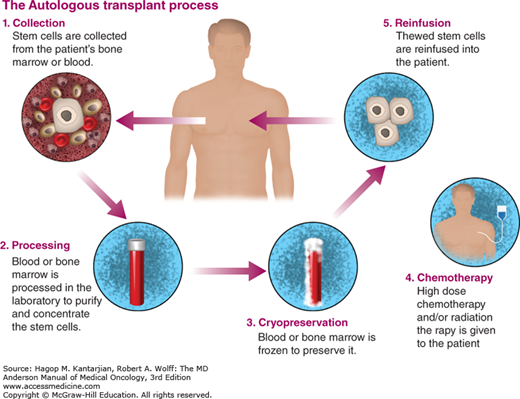
Standard chemotherapy is usually given to reduce the tumor burden prior to proceeding with HPC transplantation (Fig. 12-1). In general, the best outcomes are noted in patients with chemosensitive disease, in complete remission (CR), or with minimal tumor burden at time of transplantation.
Bone marrow is collected via multiple aspirations from the posterior-superior iliac crest in a sterile environment (usually a surgical operating room) while the patient is under anesthesia. Ideally, HPCs should be collected while the patient’s marrow is normocellular and uninvolved by the malignancy. Currently, bone marrow HPC collection is rarely, if ever, done for the purpose of autologous transplantation because HPCs can be collected from peripheral blood and engraft more rapidly when collected this way. Hematopoietic progenitor cells are normally infrequent in the blood but are mobilized into the blood during the recovery after chemotherapy and following treatment with granulocyte colony-stimulating factor (G-CSF). Peripheral blood progenitor cells (PBPCs) are collected using apheresis with continuous-flow cell separation. One to four daily apheresis sessions are usually required to achieve the minimal target CD34+ cell dose (at least 2 × 106/kg). The collected PBPCs are subsequently cryopreserved and stored.
Multiple factors have been shown to predict poor success in mobilization and collection, including advanced age; amount of preceding chemotherapy; presence of marrow-infiltrating disease; history of pelvic radiation; prior exposure to certain drugs (melphalan, carmustine, bendamustine); low premobilization platelet counts; short intervals from last chemotherapy cycle to mobilization; inadequate chemotherapy-mobilizing regimens or low-dose G-CSF (1); and previous treatment with four or more cycles of lenalidomide (2). Peripheral blood CD34+ cells more than 10/μL are usually necessary for an adequate collection. Plerixafor has become available as a second PBPC-mobilizing agent, effective for “poor mobilizers,” acting synergistically with G-CSF (3).
Autologous transplants are most effective in diseases where up to a three- to fivefold dose escalation of myelosuppressive drugs or radiation leads to a markedly increased cytotoxic effect against the malignancy. The most commonly used drugs are alkylating agents.
The most common cause of failure after autologous HPC transplantation is relapse of the underlying malignancy. This usually occurs because of inadequate systemic cytoreduction, although it may also be caused by reinfusion of malignant cells contaminating the transplant infusion. The optimal HDC regimen is disease specific. Various approaches are being studied to improve the final outcomes, including novel chemotherapy agents (4,5), monoclonal antibodies (6), chemoradiotherapy (7,8), and targeted radiation treatments. “Tandem” autologous HPC transplants have been studied in myeloma and germ-cell tumors (9). In chemosensitive malignancies, increasing the total dose of therapy may markedly improve the tumor response, but this increases the severity of side effects. Thus, novel HDC regimens must be developed in carefully designed clinical trials to provide the optimal therapeutic index.
Stem cells are infused intravenously after HDC is eliminated from the patient’s bloodstream, usually 1 to 3 days after completion of the treatment. The cells circulate transiently and home to the bone marrow. Hematopoiesis is restored within a few weeks. Hematopoietic recovery is most rapid with infusion of high doses of CD34+ cells. Most centers require a minimum 2 × 106/kg CD34+ cells per kilogram from peripheral blood (10). Neutrophils recover typically in 7 to 10 days and platelets recover in 10 to 14 days after infusion.
Patients usually receive G-CSF or other hematopoietic growth factors to accelerate neutrophil recovery. Patients are routinely prescribed prophylactic antibiotics and antiviral and antifungal therapy for prevention of infection during the initial phase of marrow engraftment and hematologic recovery.
CONTROVERSIES
The relative role of allogeneic versus autologous transplants has been long debated for specific hematologic malignancies. Autologous HPC transplantation is a process that carries less overall morbidity and mortality because the reinfused cells are not subject to immunologic rejection and do not produce graft-versus-host disease (GVHD). On the other hand, there is a risk of tumor contamination of the autologous HPCs. Some clinical studies have shown that autograft contamination is correlated with shortened disease-free survival (DFS) (11) and that the presence of tumor cells or their inadequate purging in autologous samples may correlate with the extent of the disease. Gene-marking studies in neuroblastoma showed that persistent cancer cells in the autograft can contribute to systemic relapse. The risk of contamination of the graft is higher in patients with uncontrolled tumors and known marrow involvement. Perhaps more important, there is no evidence that immune-mediated graft-versus-tumor effect associated with allogeneic HPC transplantation occurs with autologous transplants.
COMPLICATIONS OF HIGH-DOSE CHEMOTHERAPY
High-dose chemotherapy produces profound pancytopenia that usually lasts from 7 to 10 days. Infectious complications can occur in the neutropenic period (ranging from febrile neutropenia to life-threatening septic episodes) and up to 6 months or more posttransplant in the case of Pneumocystis jirovecii, fungal infections, or zoster reactivation. Antibacterial prophylaxis with a fluoroquinolone, antiviral prophylaxis with acyclovir/valacyclovir, and antifungal prophylaxis with fluconazole are generally started at the time of stem cell infusion and continued until recovery from neutropenia. Pneumocystis prophylaxis (eg, trimethoprim/sulfamethoxazole) is usually started day 30 posttransplant for at least 6 months. Antiviral prophylaxis (acyclovir, valacyclovir) are given for 6 to 12 months (12).
High doses of chemotherapy may produce major toxicities in nonhematopoietic tissues. The oral mucosa and the gastrointestinal tract are generally the most sensitive tissues. The lungs, heart, liver, brain, and kidneys are less commonly affected and are generally affected in heavily pretreated patients or those with comorbid conditions. Overall, up to 4% of patients die from regimen-related toxicities or infections. This rate of treatment-related mortality makes autologous transplantation substantially safer than allogeneic transplantation.
Toxic interstitial pneumonitis occurs in up to 40% of patients undergoing high-dose therapy. It has been described with several different chemotherapy agents, such as carmustine (13,14) and total-body irradiation (TBI) (15). It is particularly common in patients previously treated with mediastinal radiotherapy (16,17). Steroids constitute the mainstay of treatment of interstitial pneumonitis.
Cardiac toxicity is uncommon. It can be seen after high doses of cyclophosphamide or melphalan. Prior radiation therapy to the mediastinum or left chest wall and advanced age are also predictors of an increased risk of cardiac complications (18).
Central nervous system complications are rare, but dementia and leukoencephalopathy have been described as complications of HDC. Hypothyroidism frequently occurs 6 months to 2 years after therapy.
Hemorrhagic cystitis, after high-dose cyclophosphamide or ifosfamide, is uncommon and can be effectively prevented with MESNA (2-mercaptoethanesulfonate) (19,20).
Sinusoidal obstruction syndrome (SOS) (formerly known as veno-occlusive disease or VOD) of the liver is one of the most feared complications associated with HDC. Its clinical syndrome is characterized by fluid retention, hyperbilirubinemia, and hepatomegaly, which can be painful (21). Its severity depends on the presence of multiorgan failure and the rapidity of bilirubin rise. Mild cases are often self-limited; severe cases are fatal in more than 80% of patients. Several factors predispose to the development of SOS, including prior liver impairment, older age, and iron overload. The use of oral busulfan and TBI have been most commonly associated with SOS, particularly if used along with cyclophosphamide (22). Andersson et al pioneered the use of intravenous busulfan, which decreases the risk of SOS compared with the oral drug formulation (23). Pharmacokinetic-guided dosing of busulfan has further decreased the incidence of SOS. Systemic anticoagulant and thrombolytic therapies to treat SOB are ineffective and are associated with major bleeding complications. Defibrotide has emerged as a promising therapy for severe cases of SOS but has not received approval by the Food and Drug Administration yet. Supportive care plays a crucial role, using diuretics and sodium restriction, avoidance of hepatotoxins, oxygen support, and renal replacement therapy if needed.