INTRODUCTION
For patients with hematologic malignancies, allogeneic stem cell transplantation (alloSCT) provides potentially curative therapy over standard therapeutic approaches in patients whose disease is incurable with standard chemotherapy. With the development of novel techniques of transplantation, including reduced-intensity conditioning (RIC) and management of transplant-associated toxicities, increasing numbers of patients with advanced age or comorbidities can undergo this potentially lifesaving procedure. According to the most recent statistics from the Center for International Blood and Marrow Transplant Research (CIBMTR), a consortium that collects data from over 400 transplant centers worldwide, over 9,000 patients received alloSCT in 2011, up from 7,500 in 2001 (1). Given the increasing frequency of stem cell transplants worldwide, practitioners need to be familiar with the key concepts regarding the indications, procedures, and management of this technique. This chapter reviews the current state of alloSCT, with particular attention to strategies utilized at the MD Anderson Cancer Center (MDACC). The specific indications for alloSCT are briefly discussed, but a more in-depth discussion of indications is found in chapters covering specific disease states.
Allogeneic stem cell transplantation was first explored in humans in the late 1950s and early 1960s based on observations in animal models that lethal myelosuppression induced by total-body irradiation (TBI) could be overcome by the infusion of healthy, untreated bone marrow (2). The initial experience was limited to patients with terminal leukemia or severe marrow failure states resulting from radiation exposure or disease. Almost all of these early patients died from complications of graft failure, graft-versus-host disease (GVHD), infections, or primary disease (3). The first successful allogeneic bone marrow transplant was reported in 1968 in a patient with severe combined immunodeficiency (4). Since these initial experiences, alloSCT has been used to treat thousands of patients with historically incurable diseases.
In the early days of the field, it was thought that the curative effect of alloSCT was provided primarily by the high doses of chemotherapy and radiation administered, and that the donor bone marrow simply allowed for hematopoietic recovery in an adequate period of time. However, it was later determined that the stem cell graft, and associated donor immunity, was responsible for the curative potential of alloSCT. This graft-versus-tumor (GVT) effect was first demonstrated in a landmark study published in 1990 (5). This study showed decreased rates of relapse in patients who experienced GVHD, an auto-immune syndrome associated with human leukocyte antigen (HLA) mismatch between the donor and recipient. Patients who experienced GVHD had lower rates of relapse compared to patients who either did not experience GVHD or received T-cell-depleted/syngeneic grafts. Patients with GVHD had a strong GVT effect, suggesting that similar mechanisms underlie the immunologic biology of both processes. However, it does not appear that GVHD is necessary for the GVT effect. Separating the adverse effects of GVHD from the therapeutic effect of the GVT effect remains the goal of current efforts to maximize the benefit of alloSCT.
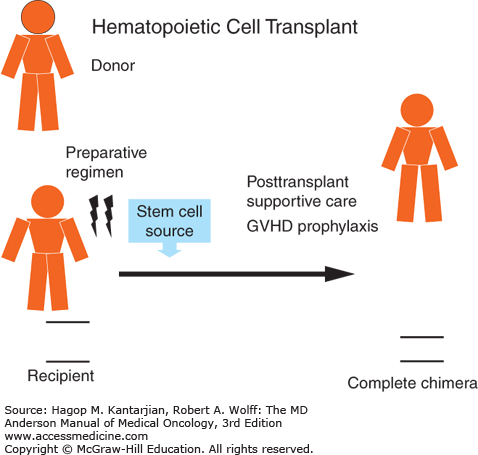
The primary components of all allogeneic hematopoietic transplants are schematically represented in Fig. 13-1 and include recipient, donor, preparative regimen, stem cell source, prophylaxis against GVHD (including posttransplant immune suppression), and posttransplant supportive care. Successful allografting depends on careful consideration of all these components in an effort to minimize the risks of potentially fatal posttransplant complications.
COMPONENTS OF ALLOGENEIC STEM CELL TRANSPLANTATION
Allogeneic stem cell transplantation is indicated for a variety of hematologic disorders, although the indication for alloSCT is dependent on the disease, remission status, stage, cytogenetics, and molecular markers in an individual patient. According to the CIBMTR database, the most common indications for alloSCT worldwide are, in decreasing order: acute myelogenous leukemia (AML), acute lymphoblastic leukemia (ALL), myelodysplasia/myeloproliferative disease, nonmalignant disease, non-Hodgkin lymphoma (NHL), other malignant disease, chronic lymphocytic leukemia (CLL), aplastic anemia, chronic myelogenous leukemia (CML), Hodgkin lymphoma (HL), and multiple myeloma (MM).
The most important recipient-related factors are disease status at transplant and comorbidity status. Over the past 5 years, two risk scores have been developed that enable clinicians to determine who the best transplant recipient is and when transplant should occur. The first is the Armand Disease Risk Index (DRI) (6). This analysis identified four risk groups (low, intermediate, high, very high) based on disease type (AML, ALL, cytogenetic classification, etc.) and disease stage (remission status, complete remission [CR], partial remission, induction failure, etc.). Patients in the low, intermediate, high, and very high risk groups had 4-year overall survival (OS) rates of 64%, 46%, 26%, and 6%, respectively, with higher rates of relapse in the higher-risk groups. This scoring system was validated in multivariate analysis as an independent predictor of OS, progression-free survival (PFS), relapse, and nonrelapse mortality (NRM). Subsequent investigators have confirmed the outcomes associations with DRI as well (7). The second important tool utilized in selecting patients for alloSCT is the comorbidity index (Hematopoietic Cell Transplantation-Comorbidity Index, HCT-CI), developed by Sorror et al (8). Patients’ comorbidities were analyzed to determine significant indicators of NRM. Patients with a HCT-CI score of 0, 1 or 2, and 3+ had 2-year NRM of 14%, 21%, and 41%, respectively. Increased pretransplant HCT-CI score has also been shown to predict the severity and mortality of acute GVHD (aGVHD) (9). A recent publication from the same group demonstrated that age greater than 40 years combined with the HCT-CI into a “composite comorbidity/age index” correlated with increased NRM for patients with a combined score of 3 or 4 (1 point was added to the HCT-CI for age greater than 40 years) (10). The results of this study need to be validated, but the data suggest that the HCT-CI/age index should be considered as part of the evaluation of any patient undergoing transplant. The HCT-CI score can be easily calculated using a validated web-based application (http://www.hctci.org).
At MDACC, all patients undergo an extensive evaluation prior to alloSCT. This includes a complete history and physical, pulmonary function tests (PFTs), echocardiogram, electrocardiogram (ECG), complete blood cell count, and chemistries. The performance status and HCT-CI are documented for each patient. Patients with a HCT-CI score of 0 to 2 are typically considered candidates for myeloablative transplant regimens, whereas patients with HCT-CI scores of 3 or greater are considered for reduced-intensity regimens. Regimen intensity is discussed further in the chapter. There is no specific cutoff for age in evaluating a patient for transplant; rather, a patient’s HCT-CI, DRI, age, disease status, family and social support, and personal wishes are all taken into consideration when deciding on proceeding with transplant. A personalized multidisciplinary approach, with a thorough discussion of the risks and benefits of alloSCT, should be undertaken for each patient.
Most allografts are performed using hematopoietic progenitor cells obtained from an HLA-identical sibling. Transplants using cells procured from volunteer donors, mismatched family members, and cord blood are rapidly becoming more common. The most important factor in selecting an allogeneic donor is HLA compatibility, which is determined by HLA typing. The HLA compatibility is the single most important determinant for the occurrence of severe GVHD after alloSCT.
The HLA system is encoded by a series of genes on chromosome 6. For SCT HLA-A, HLA-B, HLA-C, and HLA-DR are routinely evaluated (11). In its strictest sense, HLA identity means that the donor and recipient are matched for the amino acid sequence encoded by all HLA loci. Identity is assumed in the setting of related transplant when segregation analysis demonstrates that the donor and recipient have inherited the same maternal and paternal haplotypes (genotypic HLA identity). Otherwise, HLA identity can be verified only by sequencing all HLA loci (phenotypic HLA identity), which is impractical and rarely done.
It is important to note that conventional typing techniques detect a limited number of HLA polymorphic sequences. Therefore, “HLA matched” may not actually be “HLA identical.” Conventional serologic typing is based on the complement-dependent microlymphocyto-toxicity test and uses selected HLA-specific alloantisera or monoclonal antibodies to identify HLA antigens. A mismatch between cross-reactive antigens is considered minor, whereas a mismatch between non-cross-reactive antigens is considered major. For related patient–donor pairs, a single minor mismatch may be of little biological significance. Molecular typing relies on polymerase chain reaction (PCR) amplification of specific gene segments and can be performed (a) at a level corresponding to the specificities identified by serology (low resolution), (b) at a level where a limited number of alleles are possible (intermediate resolution), or (c) at a level where the specific allele is identified (high resolution). Sequence-based HLA typing is the most precise technique available.
At MDACC, we perform high-resolution typing at HLA-A, HLA-B, HLA-C, DR, DQ, and DP for all related and unrelated donor transplants. Only matching at HLA-A, -B, -C, and DR has been associated with improved survival (12). However, patients with DP mismatch have decreased relapse rates, although this is negated by increased GVHD rates. Patients are considered “10/10” if they have identical tissue types for both alleles HLA-A, -B, -C, DR, and DQ, resulting in 10 alleles total. In general, the order of preference at MDACC is matched-related-donor (MRD) sibling, 10/10 matched-unrelated donor (MUD), followed by either cord blood or haploidentical transplantation. The best alternative donor source has not been determined and is the subject of a randomized clinical trial (Bone and Marrow Transplant Clinical Trials Network [BMT CTN] 1101). Discussion of alternative stem cell sources is covered in detail in their respective chapters.
Once identified, the donor must undergo a thorough medical evaluation to determine that (a) he or she may donate safely, (b) the cells will be adequate for the recipient, and (c) the donor understands the risks and benefits of the procedure and provides cells voluntarily.
Preparative regimens are high doses of chemotherapy or TBI administered prior to stem cell infusion with the dual purpose of eradicating the underlying malignancy and inducing a state of immune tolerance that allows for donor cells to engraft and expand. This second effect ultimately gives rise to the GVT effect mediated by donor T cells, which was initially discovered when using donor leukocyte infusions (13,14). Preparative regimens can be categorized into myeloablative conditioning (MAC) versus RIC. RIC can be defined using the CIBMTR criteria: reversible myelosuppression without stem cell support, typically resulting in mixed chimerism in a significant proportion of patients, accompanied by a somewhat decreased rate of nonhematologic toxicity (15). The choice between MAC and RIC regimens is based on the underlying disease, the patient’s age and comorbid condition(s), and the physician’s preference.
Myeloablative conditioning regimens can generally be divided into chemotherapy-based protocols and TBI-based protocols. Total-body irradiation is both immunosuppressive and myeloablative. Single-dose TBI is associated with greater normal-organ toxicity, particularly pulmonary, compared with fractionated regimens (16). Therefore, most modern regimens deliver a total dose of 10 to 15 Gy using a variety of fractionation schedules. There is some evidence that higher total doses of TBI may be more effective at preventing relapse, but these benefits are offset by increased NRM. High-dose cyclophosphamide (Cy), a potent immunosuppressive agent, is often given as 60 mg/kg IV on two consecutive days prior to fractionated TBI as part of a Cy-TBI regimen. Although efficacious, TBI is associated with a number of short- and long-term complications, including secondary malignancies, retarded growth and intellectual development, cataracts, and endocrine dysfunction.
The toxicities of TBI-based strategies led to the development of radiation-free conditioning regimens. The most commonly used chemotherapy is the combination of busulfan and cyclophosphamide (BuCy). Busulfan was traditionally administered orally as 4 mg/kg/d divided into four daily doses and given on each of four successive days (total dose, 16 mg/kg), followed by Cy as depicted previously over 2 to 4 days. This was often complicated by excessive toxicity, including veno-occlusive disease (VOD)/sinusoidal obstruction syndrome (SOS), due to the unpredictable bioavailability of oral Bu combined with an adverse interaction with Cy. An intravenous formulation of Bu subsequently has allowed for once- or twice-daily dosing with more predictable systemic drug exposure, especially when coupled with pharmacokinetic targeted dose monitoring (TDM, or “targeted Bu”) (17). The combination of intravenous Bu and Cy was better tolerated and improved relapse and survival rates (18). Further large-scale comparisons of TBI-based versus intravenous Bu-based conditioning in North America and Europe conclusively demonstrated that intravenous Bu-based conditioning is safer and more efficacious than both TBI- and oral Bu-based conditioning for myeloid disorders (19,20,21). These and recently completed studies at MDACC led to targeted Bu becoming the new standard of care when Bu is used as part of an MAC regimen (22).
Cyclophosphamide and its metabolites contribute to the development of hemorrhagic cystitis posttransplant and to serious, life-threatening or lethal liver toxicity of the preparative regimen. We sought to substitute Cy with an immunosuppressive agent that would not utilize the GSH/GST and the hepatic cytochrome P450 (CYP450) pathways in its metabolism. The choice came to rely on nucleoside analogues, initially fludarabine (Flu), which replaced Cy in many conditioning regimens. Initial studies with the combination of Flu and Bu (BuFlu) produced high engraftment rates with low levels of toxicity, and this is now considered a standard of care MAC regimen at both MDACC (23,24) and other major transplant centers in North America. In these variant regimens, Flu is typically administered at a dose of 40 to 50 mg/m2 on days (-7) -6 to -3, and each Flu dose is followed by intravenous Bu at a dose of 130 mg/m2 over 3 hours on days -6 to -3, adjusted to an average daily systemic exposure (represented by an AUC of 4,000 to 6,000 μmol-min, or total course AUC of 16,000 to 24,000 μmol-min). Alternative regimens under investigation at MDACC include Bu, Flu, and clofarabine, which may result in increased antileukemic activity (25). While both Cy-TBI and the variant BuCy regimens provide additive cytotoxic activity from the agents included in the regimen, the beneficial synergistic interaction between the nucleoside analogue Flu and the alkylating agent Bu is highly dependent on optimized sequencing. It is critical that Flu precedes intravenous Bu, both of which are administered once daily (26). A summary of conditioning regimens, with disease-specific information and outcomes, can be found in Tables 13-1 and 13-2.
| Disease Type | RIC MAC | Conditioning Regimen | OS | PFS | GVHD Rates |
|---|---|---|---|---|---|
| Relapsed/refractory Hodgkin lymphoma | RIC | Fludarabine/melphalan | 64% | 32% | aGVHD grade II-IV: 28% |
| RIC | Gemcitabine, fludarabine/melphalan | 87% (18 months) | 49% (18 months) | NR | |
| Relapsed CLL/NHL | RIC | Fludarabine, bendamustine, rituximab | 90% (2 years) | 75% (2 years) | aGVHD grade II-IV: 11% Extensive cGVHD: 26% |
| Relapsed follicular lymphoma | RIC | Fludarabine, cyclophosphamide, rituximab | 85% | 83% | aGVHD II-IV: 11% Extensive cGVHD: 36% |
| Acute lymphocytic leukemia | MAC or RIC | Busulfan (AUC 4,000 μM/min RIC, AUC 5,500 μM/min MAC), clofarabine | 67% | 54% | aGVHD II-IV: 38% Extensive cGVHD: 7% |
| Philadephia chromosome–positive ALL | MAC or RIC | BuMel, Flu/Mel, or TBI based (multiple regimens) | 33% | 31% | aGVHD II-IV: 30% |
| Disease Type | RIC MAC | Conditioning Regimen | OS | PFS | GVHD Rates |
|---|---|---|---|---|---|
| Chronic myelogenous leukemia (TKI resistant)b | MAC/RIC | MAC:BuFlu, BuFluClo RIC:FluMel, BuFlu based (±TKI, rituxan), others | Mutated: 44% Unmutated: 76% | Mutated: 36% Unmutated: 58% | aGVHD II-IV: 25% Extensive cGVHD: 20% |
| Acute myelogenous leukemia/MDS/CML | MAC | Busulfan, fludarabine | 68% (CR1) 42% (CR2) 30% (PD) | 68% 42% 30% | aGVHD II-IV: 40% cGVHD: 43% |
| MAC | Clofarabine, fludarabine, once-daily busulfanc | 50% | 50% | aGVHD II-IV: 39% | |
| Acute myelogenous leukemia/MDS | RIC | Fludarabine, melphalan | 71% | 68% | aGVHD II-IV: 25% Extensive cGVHD: 29% |
| Myelofibrosisd | Busulfan (dose dense), fludarabine | 75% | 61% | aGVHD II-IV: 22% cGVHD: 39% |
According to the CIBMTR, in 2011 approximately 3,200 RIC transplants were performed, two-thirds of which were in patients over the age of 50. The underlying principle of RIC preparative regimens lies in the immunosuppressive properties of the conditioning regimen to facilitate engraftment, which utilizes immunocompetent donor cells to establish a GVT effect rather than conditioning chemotherapy to attain disease control (27). The intensity of the conditioning therapy is generally not enough to control the malignant disease but is sufficient to suppress recipient immunity, promote engraftment, and trigger a subsequent GVT effect. Patients with a history of extensive pretransplantation chemotherapy, prior radiotherapy to the chest or abdomen, prior malignancy, or underlying dysfunction of heart, lung, liver, or kidneys are at increased risk for NRM. This was reflected in the Sorror HCT-CI, in which patients were found to have higher NRM when the HCT-CI was greater than 3. A recent analysis by Sorror et al suggested that a composite HCT-CI/age comorbidity index may be employed, where patients with a score less than 3 had improved 2-year survival and could tolerate MAC or RIC regimens, and patients with HCT-CI of 3 or greater may benefit from a lower-intensity, nonmyeloablative (NMA) regimen, although this hypothesis needs to be prospectively validated. At MDACC, patients with an HCT-CI of 3 or greater generally do not receive standard MAC and are offered an RIC program.
Multiple RIC regimens exist, and they vary in intensity from nearly myeloablative to completely NMA regimens. Like MAC regimens, RIC regimens can be with chemotherapy only or be combined with TBI. Preclinical work in a canine model established that doses as low as 2 Gy were sufficient to allow engraftment of donor stem cells when used in conjunction with postgrafting immunosuppression (28). A similar regimen piloted in patients with hematologic malignancies who were more than 50 years old or had significant medical contraindications to standard transplantation demonstrated a lower degree of treatment-related toxicity and a high rate of mixed chimerism by day 28 (29). A substantial proportion (20%) of patients experienced graft rejection. A later modification to the conditioning regimen included Flu, which improved donor chimerism and decreased graft rejection (30). Subsequently, Bu 3.2 mg/kg IV was added to decrease the risk of disease relapse (31). Other chemotherapy-based regimens include Bu/Flu, Flu/carmustine/melphalan (Mel), Flu/cytoxan/TBI, and Flu/Mel (7,32,33,34,35). The RIC regimen typically used at MDACC, and implemented at many transplant centers, is the Flu/Mel regimen, utilizing Flu 25 mg/m2 over 5 days and Mel 140 mg/m2 (33). A less-ablative regimen utilizes only 100 mg/m2 of Mel. A summary of conditioning regimens, with disease-specific information and outcomes, is shown in Tables 13-1 and 13-2.
Historically, stem cells used for transplantation were obtained through the harvest of bone marrow cells from the posterior iliac crests of normal donors. Approximately 150 aspirations are necessary to yield 10 to 15 mL/kg of bone marrow, and the procedure is performed under general anesthesia, is associated with a low incidence of complications, and generally is done on an outpatient basis (36). Common sequelae of bone marrow harvest include pain and transient postoperative fever. Life-threatening complications occur in less than 1% of patients. In general, total nucleated cell (TNC) doses of 1 to 4 × 108 cells per kilogram of recipient weight are required to induce stable engraftment in patients treated with chemotherapy or TBI. Marrow cell dose is an important prognostic factor for survival in both sibling and unrelated donor transplantation (37).
Since the early 1990s, the use of peripheral blood-derived stem cells (PBSCs) has become increasingly common. The PBSCs are collected by utilizing recombinant growth factor granulocyte colony-stimulating factor (G-CSF), given for 4 to 6 days at doses of 6 to 16 μg/kg/d, to mobilize hematopoietic stem cells into the peripheral blood, where they may be collected by one or more leukapheresis procedures. A higher dose of CD34+ cells is required when utilizing PBSCs as a stem cell source, with CD34+ cells infused at an average of 2 to 10 × 106/kg, 4 × 106/kg being the standard of care at MDACC, with a minimum dose of 2 × 106/kg.
The rationale for using PBSCs was derived from studies in the autologous setting demonstrating accelerated recovery of hematopoiesis as compared with traditional bone marrow transplantation (BMT) (38,39). Numerous studies, including randomized trials comparing peripheral blood and bone marrow, have now confirmed the efficacy and safety of this approach (40). For donors, this has eliminated the risks and morbidities associated with general anesthesia and bone marrow harvest. The PBSC transplantation offers a number of advantages to the recipient. The G-CSF–mobilized PBSCs are enriched for pluripotent CD34+ hematopoietic progenitors when compared with marrow grafts (41). This has shortened the duration of neutropenia and thrombocytopenia in recipients by approximately 5 days, with median time to engraftment of 14 days (40).
Despite the benefits of more rapid engraftment with PBSCs, a major concern in many studies was an increased rate of GVHD in patients receiving PBSCs. In a major recent study, the incidence of chronic GVHD (cGVHD), but not aGVHD, was higher in recipients of MUD transplants from peripheral blood as opposed to marrow grafts (53% vs 41%, P = .01), although there was no difference in OS (51% vs 46%, P = .29) (42). The incidence of graft failure was higher in the bone marrow group versus the PBSC group (9% vs 3%, P = .002). At MDACC, the standard of care for MRDs is PBSC harvest. For MUDs, the standard of care is bone marrow harvest given the increased rates of cGVHD with PBSC harvest. However, for patients who have marrow failure syndromes, particularly myelofibrosis, or for whom rapid engraftment is necessary, PBSC is sometimes utilized.
Umbilical cord blood (UCB) is increasingly utilized as a stem cell source, and its use is discussed in the chapter on alternative donors.
Graft-versus-host disease is a multisystem immunologic disorder that greatly impacts long-term outcomes, including NRM, and quality of life after alloSCT. It continues to represent one of the major obstacles for allogeneic transplantation. The disease is mechanistically linked to the desired outcome of the GVT effect—a concept now universally accepted as a major reason for the success of allogeneic transplantation in reducing disease relapse. Diagnosis and treatment of GVHD are discussed in the section on posttransplant complications. Here, we will discuss the methods of prophylaxis against GVHD.
Two forms of GVHD, acute and chronic, are commonly distinguished based on the timing of occurrence and the clinical manifestations. Chronic GVHD typically presents more insidiously later in the posttransplant period and is associated with sclerosis of the skin, oral ulcerations, xerostomia/xeropthalmia, or bronchiolitis obliterans (BO). However, an “overlap” syndrome, with features of both acute and chronic GVHD, has increasingly been described.
Given the underlying morbidity and mortality associated with GVHD, multiple approaches to reduce the risk of both aGVHD and cGVHD have been implemented. These include posttransplant immune-suppressive chemotherapy (methotrexate, MTX), posttransplant Cy [post-Cy]), immune suppression through T-cell inhibition via calcineurin inhibitors (CNIs) (tacrolimus, cyclosporine), and T-cell depletion (ex vivo and in vivo with antithymocyte globulin [ATG]).
The two CNI inhibitors, tacrolimus and cyclosporine A (CsA), are closely related and are used in combination with MTX for MUDs and MRDs. Cyclosporine prevents T-cell activation by inhibiting the production of interleukin 2 (IL-2) and the expression of IL-2 receptors. It was discovered in the 1980s, initially in the context of aplastic anemia therapy, then in leukemia, that the combination of CsA and MTX was superior in reducing GVHD rates compared with CsA alone (43). The combination of CsA with MTX is initiated intravenously 1 to 2 days prior to stem cell infusion and converted to oral twice-daily dosing when possible. The risk of aGVHD increases when cyclosporine blood concentrations drop below a target level, typically 200 to 400 ng/mL. Cyclosporine has myriad drug interactions, which may result in fluctuating levels. Tacrolimus (FK506) is a macrolide lactone closely resembling cyclosporine in mechanism of action, spectrum of toxicities, and pharmacologic interactions. It was found to be effective in the prevention of GVHD from MRDs and MUDs in the 1990s (44). In a phase III trial, the combination of tacrolimus and MTX prophylaxis was superior to cyclosporine and MTX in reducing grade II to IV aGVHD and thus is the standard of care at MDACC (45). Tacrolimus is initiated at day -2, with goal levels of 6 to 10 ng/mL. A third agent, sirolimus, inhibits the mammalian target of rapamycin (mTOR) pathway and has been utilized as an alternative to CNI. A combination of sirolimus with tacrolimus was equivalent to tacrolimus/mini-MTX for the prevention of GVHD (46,47). At MDACC, sirolimus is typically used for patients unable to tolerate tacrolimus.
Chemotherapy approaches to GVHD prophylaxis include MTX combined with a CNI, or post-transplant cyclophosphamide (post-Cy). Chemotherapy, administered shortly after stem cell infusion but prior to engraftment, is thought to eradicate rapidly proliferating allo-reactive T cells, thus decreasing GVHD. Methotrexate at high doses has the potential for increased severity of regimen-related mucositis and delays in engraftment. These toxicities preclude full dosing of the drug. At MDACC, a modification of the cyclosporine and MTX regimen using mini-MTX was as effective as the full dose, with less toxicity (48). Methotrexate was administered on days +1, +3, +6, +11 at a dose of 5 mg/m2 in combination with methylprednisolone. Subsequently, it was determined that outcomes were identical without methylprednisolone. Thus, mini-MTX, in combination with a CNI, is the standard of care at MDACC for MRDs and MUDs.
In recent years, a calcineurin-free regimen, utilizing post-CY, based on initial success in the haploidentical setting, has become an attractive potential alternative to standard CNI/MTX prophylaxis. Initial studies in the myeloablative setting with BuCy and BuFlu demonstrated comparable rates of GVHD when administering Cytoxan at 50 mg/kg on days +3 and +4 (49,50). However, in a phase II study at MDACC, post-Cy utilized in the RIC setting resulted in increased mortality (unpublished data). Therefore, this regimen should still be considered experimental, and it is the subject of a randomized CTN trial (BMT CTN 1203).
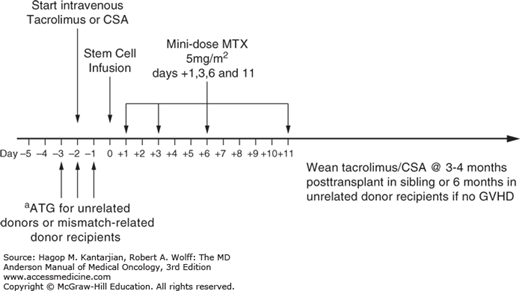
In addition to prophylaxis with CNIs and chemotherapy, T-cell-depleting approaches have demonstrated some success in decreasing the rates of GVHD, both in vivo with ATG and ex vivo with T-cell depletion. The hypothesis is that by depleting allo-reactive T cells (thought to cause GVHD), rates of GVHD will be lower. The use of ATG resulted in decreased rates of severe aGVHD and extensive cGVHD in unrelated donors (51,52). However, the use of ATG remains controversial. A meta-analysis published in 2012 from the Cochrane database showed that ATG did not improve OS, NRM, or relapse, but resulted in decreased incidence of grade II to IV aGVHD (53). At MDACC, rabbit ATG is administered on days -3 through -1 (total dose of 4 mg/kg) for MUDs, in combination with mini-MTX/tacrolimus. The MRDs receive mini-MTX/tacrolimus prophylaxis without ATG. An alternate method is ex vivo T-cell depletion prior to marrow infusion; this effectively reduces the incidence and severity of GVHD. However, T-cell depletion also results in increased graft rejection, infectious complications, and relapse rates in a disease-specific manner. Selective depletion of T-cell subsets may be more effective in reducing the risk of acute GVHD while preserving the GVT effect but is not practiced at MDACC (54,55). A summary of the approach to GVHD prophylaxis at MDACC is shown in Fig. 13-2.
POSTTRANSPLANT SUPPORTIVE CARE
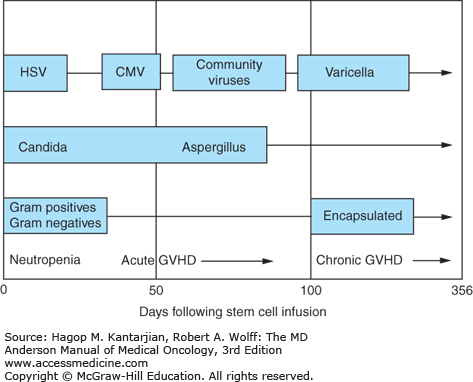
The basic principle underlying supportive care is prevention. Most transplant complications have a temporal relationship to the conditioning regimen and the transplant. Appropriate supportive care measures are therefore dependent on anticipated complications. The temporal relationship of common infectious complications is depicted in Fig. 13-3. A summary of posttransplant complications, diagnosis, and management is shown in Table 13-4 and is discussed next.
Infection prophylaxis is provided to guard against bacterial, fungal, and viral pathogens. Based on the high risk of infectious complications and associated significant morbidities, a number of prophylactic and surveillance strategies have been developed. Patients are divided into “high-risk” and “low-risk” categories prior to stem cell transplantation. Standard prophylaxis for low-risk patients (MRD, low-risk MUD with lymphoma, aplastic anemia, myelofibrosis, CML, first and second remission [CR1/CR2] leukemia) is with fluconazole, levofloxacin, and valacyclovir, with trimethoprim/sulfamethoxazole for Pneumocystis pneumonia (PCP) prophylaxis. Higher-risk patients, particularly those with known fungal infections, may be treated with posaconazole/voriconazole. A summary of infectious prophylaxis strategies, including for patients being treated for GVHD, is shown in Table 13-3 (56,57,58,59,60,61,62).
| Risk Category | Antibacterial | Antifungal | Antiviral (HSV/VZV) | PCP |
|---|---|---|---|---|
| Low risk | Levofloxacin 500 mg IV or by mouth daily starting day -1 (or when ANC <1 × 103/μL) until ANC >1 × 103/μL | Fluconazole 400 mg IV or by mouth daily until off IST | Valacyclovir 500 mg by mouth dailya starting day -1 for 1 year or until off IST OR Acyclovir 250 mg/m2 or 5 mg/kg IV piggyback every 12 hours | Bactrim DS 1 tablet by mouth daily Monday, Wednesday, Friday or Bactrim SS daily starting by day +30 for 1 year and until off IST |
| High risk | Levofloxacin 500 mg IV or by mouth daily starting day -1 (or when ANC <1 × 103/μL) until ANC >1 × 103/μL | Voriconazole 200 mg IV or by mouth starting day -1 OR Posaconazole 200 mg by mouth three times daily with food starting day -2 Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|