INTRODUCTION
Fungal and viral infections remain a significant cause of morbidity and mortality in patients with cancer. Modern management of infections in cancer requires knowledge of the epidemiology, pathogenesis, treatment, and prevention of such infections. Fungal infections range from nosocomial infections with Candida spp to endemic fungi acquired outside the hospital, such as Histoplasma capsulatum. Opportunistic fungi, especially molds, have emerged as a leading cause of death in patients with leukemia or hematopoietic stem cell transplant (HSCT) (1). Viral infections such as varicella zoster virus (VZV), herpes simplex virus (HSV), or cytomegalovirus (CMV), have been associated with increased morbidity and mortality in patients with cancer, including patients with multiple myeloma or chronic lymphocytic leukemia (CLL), and in HSCT recipients (2,3,4,5). Respiratory viruses, such as respiratory syncytial (RSV), adenovirus, and influenza, are increasingly recognized as significant pathogens in patients with cancer, particularly as molecular diagnostic methods improve. In addition, viruses such as novel influenza H1N1, West Nile virus, bocaviruses, and noroviruses have emerged as newly recognized pathogens in patients with cancer.
FUNGAL INFECTIONS
Fungal infections pose a continuing challenge for oncology patients. Exposure to fungi is common, with exposure typically occurring in the environment. Patients with cancer are susceptible not only to new infection with endemic fungi (such as Histoplasma capsulatum), but also to reactivation of latent infections. Opportunistic molds, such as Fusarium spp, Scedosprorium spp, and Zygomycetes cause devastating disease in hematologic patients. Cases of nosocomial infection due to molds are reported in the setting of hospital construction, leading to routine air sampling and filtration. In contrast, Candida spp are a common component of the patient’s or health-care workers’ endogenous microbial flora. Manifestations of infection may not present until the patient receives chemotherapy or undergoes HSCT.
Diagnosis of invasive fungal infections is problematic as well, despite increased use of fungal biomarkers (6). The skin and lungs are accessible areas for examination and biopsy as they are commonly affected by fungal pathogens. In our institution, a retrospective study of skin biopsies in patients with leukemia suggested that ulcerated or necrotic skin lesions in the context of bacteremia or fungemia were predictive of infection (7). Of note, skin biopsy revealed infection in 39% of all patients undergoing biopsy and 55% of those with severe neutropenia (7). Of patients with biopsy-proven skin infection, 39% of those infections were fungal, led by Candida species (25%), Fusarium (19%), Mucorales (13%), Aspergillus species (9%), Alternaria (6%), and Curvularia (3%) (7).
In the setting of pulmonary nodules, lung biopsy, utilizing open biopsy or computed tomographic (CT)–guided percutaneous biopsy, has also proven useful in diagnosis. Studies of CT-guided biopsy show a yield of approximately 60% for a specific diagnosis (8). In a study at our center of patients with hematologic malignancy, 34% of the specific diagnoses with CT-guided biopsy were previously unidentified infection, predominantly fungal (9). A recent retrospective study of open lung biopsy revealed 19% previously undiagnosed infectious etiologies (8). Identified organisms included 58% endemic molds (Histoplasma, Coccidioides), 14% Aspergillus species, and 7% Cryptococcus species (9).
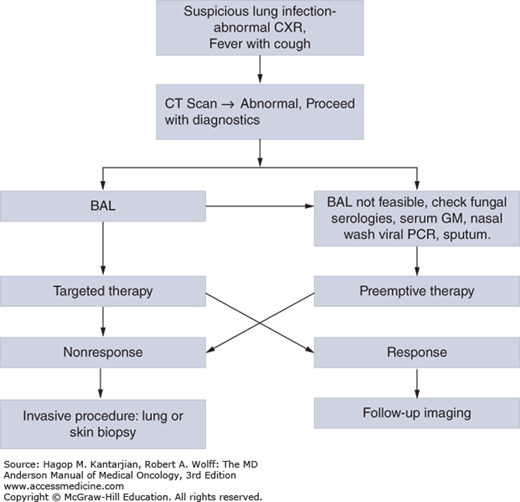
Despite these efforts, diagnosis is often a challenge if infection is more diffuse or thrombocytopenia is too severe to safely permit biopsy. Autopsy, unfortunately after all diagnostic and treatment efforts failed, has historically been an approach to ultimate diagnosis of etiology, but autopsy rates have fallen almost 90% from 0.63/100 deaths in 1989 to 1993 to 0.06/100 deaths in 2004 to 2008 at our institution (10). Diagnosis of fungal infections, often manifesting as pulmonary nodules or skin lesions, is challenging, but important, given the modification of therapy that may occur based on identification of a particular species of fungus or discovery of coinfection, malignancy, or other alternative diagnosis. Figure 51-1 suggests an approach to identification of fungal (and other) pulmonary pathogens at our institution.
Severe neutropenia, particularly prolonged, has long been associated with invasive fungal infections. Chemotherapy resulting in prolonged and severe CD4 lymphocytopenia can also result in infections similar to those seen in patients with untreated human immunodeficiency virus (HIV) or AIDS, such as cryptococcosis and reactivation of endemic fungi, including histoplasmosis and coccidiomycosis. In addition, conditioning regimens for stem cell transplant and immunosuppressives to treat or prevent graft-versus-host disease (GVHD) result in deficient cell-mediated immunity, increasing risk for invasive fungal infection (4,10). Disruption of mucocutaneous barriers predisposes to invasive candidal infection, exemplified by catheter-related bloodstream infections caused by Candida spp (11).
A recent study from Italy utilized a retrospective cohort of patients with hematologic malignancy to develop a risk prediction score for invasive fungal infections (4). The score emphasizes the central roles played by lymphopenia, relapsed or refractory malignancy, prolonged neutropenia (>10 days), and prior history of invasive fungal infection in identifying patients at highest risk of invasive fungal infection. Of interest, the authors found that posaconazole prophylaxis was exclusively beneficial to the group with highest risk of invasive fungal infection (4).
Finally, another aspect of importance is the change in flora that takes place with broad-spectrum antibacterial and antifungal therapy. The latter may result in suppression of normal bacterial flora and candidal overgrowth in the oropharynx and gastrointestinal (GI) tract. Antifungal therapy or prophylaxis may result in breakthrough infection with non–Candida albicans species, such as Candida krusei (resistant to fluconazole) (11). Aside from non–Candida albicans species, prophylaxis with non–mold active antifungals (eg, fluconazole) may predispose to infection with molds, such as aspergillosis.
CANDIDIASIS
Candidiasis remains the most common invasive fungal infection in patients with cancer. Modern medical care is increasingly complex, involving frequent antimicrobial use and device utilization that alter patient flora and disrupt the mucocutaneous barrier. Candida spp commonly arise from the patient’s endogenous flora, but rare hospital-acquired cases have been reported due to contaminated equipment, solutions, and hospital personnel. Manifestations of candidiasis range from local infection of the skin or oral mucosa to candidemia and widely disseminated infection.
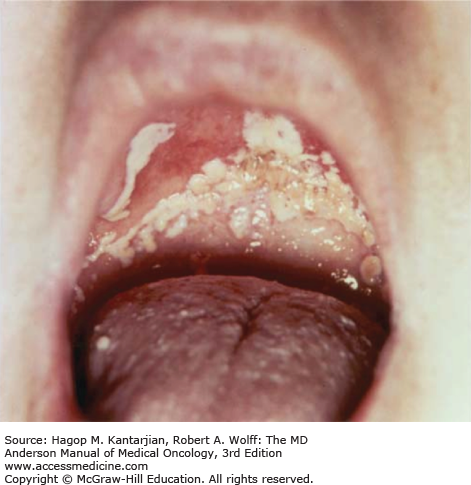
Thrush is the most common superficial candidal infection among patients with cancer, typically those with cancers involving the head and neck undergoing chemoradiation (12). Oropharyngeal candidiasis is characterized by whitish plaques on the buccal mucosa, palate, or tongue (Fig. 51-2) that may be painful if removed, exposing the erythematous base. Oral thrush may also be a manifestation of esophagitis (12). The diagnosis is commonly made clinically but is confirmed by finding yeast and pseudohyphae on scraping or culture.
Esophageal candidiasis may cause dysphagia, retrosternal pain, and odynophagia in patients with cancer (13). Serious complications of this infection can occur, including chronic esophageal strictures, bronchoesophageal fistulas, and mediastinitis. Esophagoscopy with biopsy and culture are necessary to confirm the diagnosis of candidal esophagitis (11). Unfortunately, thrombocytopenia often makes esophagoscopy challenging, so empiric therapy is often used. Esophageal candidiasis requires systemic therapy, typically utilizing fluconazole as initial therapy (11). Caspofungin or other echinocandins may be used if fluconazole fails or is not well tolerated, but it is available only as an intravenous preparation (11). Itraconazole, voriconazole, posaconazole, or amphotericin B formulations are rarely indicated for these infections (11).
Patients with cancer, as with many other hospitalized patients, may develop primary infections of the urinary tract in the settings of urinary obstruction and particularly urinary catheters. Differentiating between colonization and infection is challenging in the presence of urinary catheters. Urinalysis may be normal, and high organism counts are not sufficient to confirm infection. In febrile neutropenic patients, candiduria should be considered as a harbinger of disseminated candidiasis. Recent guidelines recommend treatment with fluconazole, with amphotericin B formulations used for resistant Candida spp (11). Of note, echinocandins fail to penetrate the urinary tract, so they should not be used in this setting. Relapse of infection, however, will be likely unless the urinary catheter is removed.
Neutropenia, presence of colonization of oropharynx and other sites, steroid use, presence of central venous catheters, and persistent fever in the setting of broad-spectrum antibacterial therapy suggest the diagnosis of candidemia (14). A study from a multicenter database demonstrated that C. albicans now represents a minority of candidemia infections (45.6%) (15). In that study, candidemia resulted in an overall 12-week crude mortality of 35.2%, with highest mortality rate associated with C. krusei (52.9%) and lowest with Candida parapsilosis (23.7%) (15). Candida parapsilosis candidemia has been associated with central venous catheters (15). Patients with C. parapsilosis, including nononcology patients from a multicenter database, were less likely to be neutropenic and immunosuppressed, perhaps explaining the lower mortality rate (15). Candida krusei has been associated with prior antifungal use, hematologic malignancy (including stem cell transplant), neutropenia, and steroid use. These host factors associated with infection suggest why C. krusei exhibits the highest mortality rate of species causing candidemia (52.9%) (15).
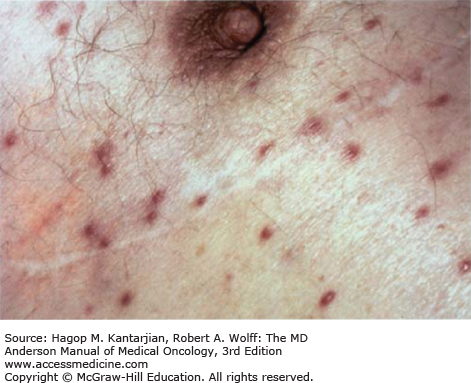
Disseminated candidiasis is difficult to differentiate from other disseminated fungal and bacterial infections. Persistent fever in the setting of antibacterial therapy and liver dysfunction may suggest consideration of disseminated candidiasis (15). In patients with cancer, disseminated candidiasis typically originates from the GI tract or central venous catheters. Dissemination affects multiple organs, such as the kidneys, heart, GI tract, lung, liver, spleen, and skin (16). Candida tropicalis is more likely to cause the characteristic skin lesions associated with disseminated candidiasis and occasionally causes a syndrome of skin lesions and painful myositis (17). Lesions may appear as clusters of pustules or larger nodules and may even develop necrotic centers similar to ecthyma gangrenosum (18). Common presentation is nontender, firm, nonblanching, raised nodules that are pink to red in color (Fig. 51-3).
The diagnosis of disseminated candidiasis may be difficult to establish because culture of the organism from sputum, urine, and feces may be positive in patients without infection. On the other hand, 40% of patients with widespread infection demonstrated at autopsy examination had multiple negative blood cultures (16). Given the challenge of appropriate diagnosis and morbidity associated with failure to treat the severely immunocompromised, empiric therapy is commonly given for those who continue to be ill in the setting of broad-spectrum antibacterial therapy.
Therapy for candidiasis includes three classes of medications: azoles (eg, voriconazole), echinocandins (eg, caspofungin), and the polyenes (eg, amphotericin B). Dosing regimens, major toxicities, and general considerations for antifungal agents are shown in Tables 51-1,51-2, 51-3, respectively. Timely appropriate therapy is necessary because the mortality rate for candidemia ranges from 24% for candidemia caused by C. parapsilosis to as high as 53% for C. krusei (15). Candida glabrata exhibits decreased susceptibility to fluconazole (14). Mortality, however, is not significantly different from C. albicans candidemia (14). Candida krusei, inherently resistant to fluconazole, is increasingly isolated in institutions where fluconazole is commonly used for prophylaxis (19). Candida lusitaniae, more commonly seen in patients with stem cell transplant or neutropenia, is of concern due to amphotericin B resistance (20).
| Drug | Loading Dose | Daily Dose | Route |
|---|---|---|---|
| D-AMB | — | 1-1.5 mg/kg | IV only |
| Lipid AMB | — | 3-5 mg/kg | IV only |
| Fluconazole | 800 mg | 400-800 mg | IV, Oral |
| Itraconazole IV | 200 mg bid × 2 days | 200 mg | IV |
| Itraconazole solution | 200 mg bid × 2 days | 200 mg | Oral |
| Posaconazole tabs | 300 mg bid × 2 doses | 300 mg | Oral |
| Posaconazole IV | 300 mg bid × 2 doses | 300 mg | IV |
| Posaconazole (susp) | — | 200 mg every 6h | Oral |
| Voriconazole IV | 6 mg/kg every 12 h × 2 doses | 4 mg/kg every 12 h | IV |
| Voriconazole tabs | — | 200 mg every 12 h (>40 kg) | Oral |
| 100 mg every 12 h (<40 kg) | |||
| Caspofungin | 70 mg × 1 dose | 50 mg | IV |
| Micafungin | — | 150 mg | IV |
| Anidulafungin | 200 mg × 1 dose | 100 mg | IV |
| Amphotericin B | Infusion related (headache, chills, hypotension, etc); nephrotoxicity; hypokalemia, hypomagnesemia; anemia |
| Fluconazole | Nausea, vomiting; headache; hepatotoxicity (rare); drug interactions |
| Itraconazole | Nausea, vomiting; headache; hepatotoxicity (rare); pulmonary edema; drug interactions |
| Posaconazole | Hepatotoxicity (rarely requires discontinuation) |
| Voriconazole | Visual disturbances; rash; nausea, vomiting; headache; hepatotoxicity; drug interactions |
| Echinocandins (eg, caspofungin) | Fever; nausea; flushing; rash; some drug interactions; phlebitis |
| Regimen | Advantages | Disadvantages |
|---|---|---|
| Amphotericin B deoxycholate (D-AMB) | Broad-spectrum activity. | Acute chronic toxicities; minimally effective in patients with neutropenia and with chronic disseminated candidiasis; Intravenous preparations only. |
| Lipid formulations of AMB | Broad-spectrum activity, reduced nephrotoxicity. Higher doses can be administered. | Only prospective randomized trial showed no advantage in efficacy over D-AMB despite higher doses. More expensive. Intravenous preparations only. |
| Fluconazole | Oral and intravenous preparation. As effective as AMB in randomized trials of nonneutropenic individuals. Minimal toxicity. More effective for chronic disseminated candidiasis. Little experience in neutropenic patients but appears to be as effective as AMB. | Variable activity against C. glabrata and C. dubliniensis; inactive against C. krusei. Some drug-drug interactions. |
| Echinocandins (eg, caspofungin) | Broad-spectrum activity. Minimal toxicity. In randomized trials, as active as amphotericin B and fluconazole. Limited experience in neutropenic patients. | No oral preparation. |
| Flucytosine | Synergistic with AMB and fluconazole. Combination of flucytosine and AMB may be superior to AMB alone for chronic disseminated candidiasis and C. tropicalis infection. | No intravenous preparation. Causes myelosuppression. Often need monitoring of serum concentrations. Emergence of resistance if used alone. |
Recently published guidelines suggest that echinocandins be utilized as first-line therapy in neutropenic patients with candidemia, with lipid formulations of amphotericin B as second-line therapy (11). Species-specific recommendations, however, are provided, given inherent differences in resistance. The guidelines emphasize that if a therapeutic approach is resulting in clinical improvement, then current therapy can be continued. For C. glabrata, an echinocandin or lipid formulation of amphotericin B is recommended (11). For infection with C. parapsilosis, an azole or lipid formulation of amphotericin B is recommended. For C. krusei, fluconazole is contraindicated due to innate resistance (11). For neutropenic patients with invasive candidiasis (but not candidemia), lipid formulations of amphotericin B, echinocandins, or voriconazole are recommended (11). In candidemia and invasive candidiasis, fluconazole may be used in patients who have no prior exposure to azoles and are not critically ill (11). If fluconazole is used, the initial recommended dose is 12 mg/kg/d. The guidelines recommend the use of lipid formulations of amphotericin B, rather than amphotericin B deoxycholate (D-AMB), to avoid nephrotoxicity (11).
Caspofungin was the first echinocandin approved by the Food and Drug Administration (FDA), showing broad-spectrum activity against Candida spp. In comparison to D-AMB in a study of invasive candidiasis (80% of which was candidemia), caspofungin showed similar outcomes with fewer adverse events (11). Of note, however, few patients were neutropenic in this study. The three currently available echinocandins (caspofungin, micafungin, and anidulafungin) are comparable in their efficacies, although only one study in patients with nononcologic candidemia directly compared micafungin and caspofungin and showed equivalent outcomes (21).
The management of catheter-related bloodstream infections is controversial. Debate exists with respect to need for catheter removal. Patients with indwelling intravascular catheters typically require them for chemotherapy or supportive care. The removal of surgically implanted catheters is particularly difficult, given thrombocytopenia is often present in this patient population and also the high costs of placement. Studies suggest a role for catheter removal, particularly if it is clear that the catheter is the source or in the setting of persistent candidemia without another source. Removal of the catheter may improve response rates and reduce duration of candidemia (11). Infection with C. parapsilosis, in particular, is associated with persistent candidemia without catheter removal (11).
In brief, Candida causes a wide spectrum of syndromes, from superficial oral candidiasis to candidemia, in patients with cancer. The therapeutic approach should take into consideration host risk factors, medication interactions, antifungal toxicities, and comorbidities when selecting a particular agent.
ASPERGILLOSIS
One of the most common and important invasive fungal infections is aspergillosis. Aspergillus spp are the most common invasive mold infections in patients with hematologic cancer (22). Aspergillus fumigatus is the species most commonly associated with infection, although Aspergillus terreus and Aspergillus flavus, more resistant Aspergillus spp, are becoming increasingly common (22). Infections are typically acquired by spore inhalation, but construction in hospitals and surrounding areas have been associated with infection (23). The most important risk factor is prolonged neutropenia (22). In patients with stem cell transplants, reported risk factors for increased mortality include poor baseline pulmonary status, high doses of steroids (≥2 mg/kg/d), disseminated aspergillosis, proved invasive aspergillosis, increased bilirubin, increased creatinine, HLA-mismatched stem cells, and invasive aspergillosis occurring 40 or more days after transplant (24). A recent retrospective study suggested that elevated serum galactomannan index (GMI), but not bronchoalveolar lavage GMI, is associated with increased mortality (25).
The most common syndrome associated with aspergillosis is pneumonia. Due to the angioinvasive nature of the infection, symptoms suggesting pulmonary involvement (eg, pulmonary embolism, pleuritic chest pain, fever, hemoptysis, and friction rub) are occasionally encountered (26). Initial chest x-ray may be unremarkable, with fever proceeding in the setting of broad-spectrum antibacterials (26).
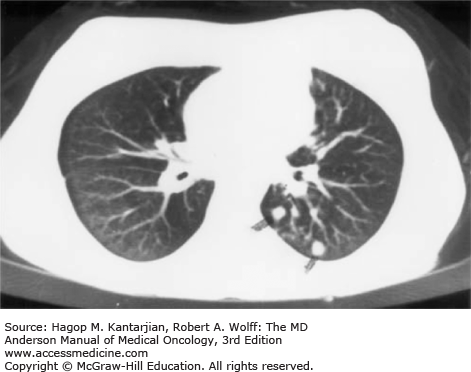
Radiologic findings are variable, with wedge-shaped infarcts, necrotizing bronchopneumonia, lobar consolidation, or diffuse infiltrates noted (26). If suspicion is high for infection, early CT scan of the thorax is essential, potentially demonstrating a halo sign (area of low attenuation surrounding a nodular infiltrate), an important early sign that disappears in 75% of cases within the first week (26) (Fig. 51-4). Analysis of radiologic studies from a clinical trial suggested that patients with the halo sign had an improved response to treatment and also improved mortality compared to those who did not exhibit the halo sign (27). Cavitation occurs as the infection progresses, with lesions often increasing in size until neutrophil recovery occurs. Differentiation from infection with other molds (ie, mucormycosis) can be difficult. A study suggested that CT of the thorax with greater than 10 nodules and pleural effusion are more often seen in pulmonary mucormycosis rather than invasive pulmonary aspergillosis (27).
Immunocompromised patients may exhibit acute sinusitis as a component of invasive diseases, occurring in 15% to 20% of neutropenic patients (28). Fever, headache, cough, epistaxis, and sinus discharge are signs and nonspecific symptoms suggestive of fungal sinusitis (28). On exam, necrotic lesions may be seen in the nose or palate (Fig. 51-5), with accurate diagnosis improved by examination and confirmed by biopsy by experienced otolaryngologists (29). Imaging of the sinuses by CT or magnetic resonance imaging scan may show opacification of the sinuses or bony destruction. Mortality may be as high as 20% in patients with leukemia in remission to 100% for those with invasive sinusitis who have refractory leukemia or are undergoing HSCT (28).
Aspergillosis as part of dissemination is discussed in the next section, although primary cutaneous infection occurs with direct inoculation. These infections are rarely associated with a central venous access device (30). The mechanism of spread is presumed to be via inoculation during catheter insertion or possibly dressing changes or application. Initially, lesions may appear as erythematous plaques, progressing to necrotic ulcers with black eschars (30). Aspergillus flavus is the most common cause of cutaneous invasive aspergillosis.
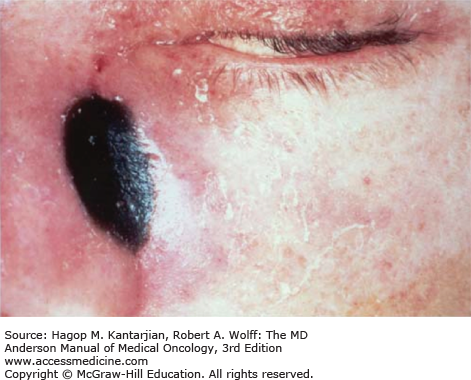
Given the angioinvasive nature of aspergillosis, hematogenous dissemination occurs in approximately 20% of patients with active hematologic malignancy or HSCT (31). Common sites of dissemination include the central nervous system (CNS), GI tract, and skin. Gastrointestinal involvement is apparent in 40% to 50% of cases, affecting the esophagus and large bowel (32). Perforation or massive hemorrhage may occur in this setting. Skin infection may also occur, evolving from erythematous plaques to ulcers that ultimately may be covered by black eschar (33). Given the broad differential diagnosis of skin lesions in this patient population, skin biopsy is critical.
A continuing challenge in management of aspergillosis is early and reliable diagnosis (26). Tissue biopsies from infected tissue may reveal invading hyphae. Paradoxically, cultures of the biopsy specimens fail to grow the fungus in over 50% of cases, although histology and pathology correlate with culture in 78% of those cases (34). Similarly, blood cultures will rarely (except in the case of A. terreus) demonstrate the organism, in contrast to fusariosis (35). Unfortunately, Aspergillus spp fail to grow well from sputum or bronchoscopy, with only 30% of biopsy-proven aspergillosis cases growing mold in sputum cultures (36). Due to these challenges of diagnosis, patients commonly receive empiric antifungal therapy while undergoing workup.
To increase the likelihood of detection of aspergillosis, various non–culture-based tests have been developed. Available tests detect circulating antigens or immune complexes. Galactomannan and 1,3-β-D-glucan are the most commonly used tests. Via a sandwich enzyme-linked immunosorbent assay used to detect the polysaccharide cell wall component of Aspergillus spp, galactomannan can be detected in the serum, and more recently bronchoalveolar lavage fluid, of infected patients (26). Sensitivity ranges from 67% to 100% and specificity from 86% to 99%, but the test has been studied primarily in patients with hematologic malignancy with profound neutropenia. The positive predictive value of this test is poor in patients with solid tumor and other cancer (26). An additional challenge occurs in interpreting a galactomannan in the context of prophylaxis with antimold activity. A recent study suggested up to 86% false-positive results for those on active antimold therapy (37).
1,3-β-D-Glucan is an integral component of the cell wall of several yeasts and fungi (26). Sensitivity ranges from 67% to 100% and specificity 84% to 100%, but false positives are noted due to cirrhosis, hemodialysis, and some chemotherapeutic agents (26).
Early diagnosis of aspergillosis utilizing the various available tools has allowed for earlier treatment, but challenges continue in assessing the impact of various treatments, given categorization of infection as proven, probable, or possible (29). Table 51-4 describes various principles for management of aspergillosis. Persistence of neutropenia is a host factor that is critical in determining the outcome of infection, regardless of therapy. In fact, in a study of patients with acute myeloid leukemia (AML), mortality was 90% in those patients who failed to recover from neutropenia (38).
Early, aggressive treatment with high doses of voriconazole or a lipid formulation of amphotericin B deoxycholate (D-AMB) Rapid tapering of dose of adrenal corticosteroids if possible Consideration for G-CSF-primed granulocyte transfusions in select cases Long-term antifungal therapy, which should be individualized based on response Debridement of necrotic tissue of localized disease (onychomycosis, sinusitis, abscess) |
Voriconazole is currently recommended as first-line therapy of invasive pulmonary aspergillosis (28). A randomized trial comparing voriconazole to D-AMB for patients with definite or probable aspergillosis showed decreased mortality in the voriconazole group (71% vs 58%). Both oral and intravenous formulations are available, with decreased nephrotoxicity compared to amphotericin B. Voriconazole does, however, cause visual disturbances, hallucinations, and liver dysfunction in a subset of patients.
Posaconazole, the newest FDA-approved azole, was previously only available as an oral solution. Posaconazole tablets are now available, with considerably improved absorption, with minimal impact from food, mucositis, or elevated gastric pH (39). Despite resultant elevated liver function tests due to dramatically increased posaconazole levels in tablet form, no clinically significant hepatotoxicity occurred (39). Posaconazole was associated with increased response to therapy and decreased mortality compared to Liposomal Amphotericin B (L-AMB) with or without caspofungin (40). In addition, nephrotoxicity and change in liver function tests were more likely in the L-AMB–containing regimens.
Lipid formulations of amphotericin B are now recommended in lieu of D-AMB, given the decreased risk of nephrotoxicity. In a randomized study of early treatment of aspergillosis in neutropenic patients, doses of 10 mg/kg/d were compared with 3 mg/kg/d with comparable outcomes (46% high dose vs 50% low dose), but the higher dose resulted in greater nephrotoxicity (32% vs 20%) (28).
Echinocandins inhibit the synthesis of β-(1,3)-D-glucan, an essential component of the fungal cell wall. A disadvantage of echinocandins is that they are available only as an intravenous preparation (28). In a noncomparative trial of 90 patients with definite or probable aspergillosis who had failed other therapy, a complete or partial response occurred in 45% (41). Responses were observed in 50% of patients with pulmonary infection but in only 26% of those with neutropenia (41).
Treatment of Invasive Aspergillosis (IA) is challenging, particularly in the setting of prolonged neutropenia. This has led to the use of combination therapy. In a recent randomized, placebo-controlled trial of HSCT and patients with hematologic malignancy, voriconazole combined with placebo or anidulafungin failed to demonstrate a difference in overall or 6-week mortality (42). In a subgroup with radiographic findings consistent with IA and elevated serum galactomannan, the mortality was significantly higher in the monotherapy group (15.7% vs 27.3%, P = .037) (42).
Antifungal agents utilized for prophylaxis against aspergillosis in high-risk patients have included posaconazole, itraconazole, voriconazole, aerosolized or nebulized formulations of amphotericin B, and micafungin (22,28). Posaconazole is the only agent currently FDA approved for the indication of prevention of invasive aspergillosis in patients with acute leukemia and high-risk stem cell transplant recipients, but significant expense continues to limit the utility of this agent (41).
CRYPTOCOCCOSIS
Cryptococci are encapsulated yeasts that have a worldwide distribution, with a dramatic increase in incidence corresponding with the advent of HIV/AIDS (43). Cryptococcus neoformans is the most common pathogen, found in pigeon excretions, with infection acquired by inhalation into the lungs. Patients with HIV/AIDS had the highest incidence of infection prior to the initiation of highly active antiretroviral therapy (43). Factors associated with cryptococcosis in patients with cancer include lymphopenia, chemotherapy, and steroid use less than 1 month prior to diagnosis (44). Those at particular risk include those with lymphoma or CLL. Risk in patients with hematologic malignancy is low because of widespread use of fluconazole and other agents for antifungal prophylaxis.
Given inhalation as the mechanism of entry, the lung typically serves as the primary site of infection. Despite this fact, less than 40% of patients present with symptoms suggestive of pneumonia (43). Symptoms may, however, include chest pain, fever, or dyspnea. Chest radiographic findings may include single or multiple nodules, airspace consolidation, reticular patterns, ground-glass opacities, cavitary lesions, and occasionally pleural effusions, with all findings unilateral or bilateral (43). Cryptococcal pneumonia can rapidly progress, resulting in higher mortality in patients with cancer. For susceptible patients, finding of this organism in a patient with chest radiography and symptoms consistent with infection is sufficient indication for therapy. In a series from a cancer center, fine-needle aspiration, bronchoalveolar lavage, and open lung biopsy had a yield of over 90% on culture (44).
Many series showed a predominance of CNS infection in patients with cancer, primarily with meningoencephalitis, but rarely with meningitis alone or with cryptococcoma. Depending on degree of immunosuppression, patients may exhibit an indolent course, with initial symptoms of fever and headache (43). As the disease progresses, symptoms may include nausea, vomiting, dizziness, somnolence, irritability, confusion, photophobia, or obtundation. Absence of nuchal rigidity (a finding exhibited in only 15% of patients) does not rule out infection (43). Patients infected with Cryptococcus gatti can include those with no apparent immunosuppression but with high predeliction for CNS involvement (43). In patients with CNS disease, findings include elevated opening pressure, decreased glucose, and high protein concentration. Leukocyte count may also be elevated, with lymphocyte predominance (44). Available diagnostic tests include India ink, serum cryptococcal antigen, and fungal culture. India ink detects 50% of infections, whereas serum cryptococcal antigen is positive in 90% of cerebrospinal fluid (CSF) and 70% of blood in patients with CNS infection.
Multiple organs, including the liver, prostate, eyes, skin, and bone, may serve as sites of dissemination. Serum cryptococcal antigen has greater than 90% sensitivity and specificity for invasive cryptococcal disease (43). Skin lesions, present in only approximately 10% of patients, tend to be painless and located on the face, neck, and scalp (43). The lesions may appear as papules, plaques, ulcerations, acneiform eruptions, lesions, or even draining sinuses. With wide use of antifungal prophylaxis, skin, soft tissue, and osteoarticular lesions appear to be less common (43).
Treatment of cryptococcal disease depends largely on site of infection. Combination of D-AMB with flucytosine is the traditional approach of choice for severe pulmonary cryptococcosis and CNS disease in non–HIV-infected immunocompromised patients as induction therapy (43). Flucytosine is available only as an oral preparation with myelosuppressive toxicity (see Tables 51-1
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree