Testis cancer
Christian Kollmannsberger, MD, FRCPC  Craig R. Nichols, MD
Craig R. Nichols, MD  Siamak Daneshmand, MD
Siamak Daneshmand, MD  Eric K. Hansen, MD
Eric K. Hansen, MD  Christopher L. Corless, MD, PhD
Christopher L. Corless, MD, PhD  Bruce J. Roth, MD
Bruce J. Roth, MD  Lawrence Einhorn, MD
Lawrence Einhorn, MD
Overview
Cancer of the testis is a relatively uncommon disease, accounting for approximately 1% of all cancers in males. However, it represents a highly curable neoplasm, the incidence of which is focused on young patients at their peak of productivity. Curative treatment of disseminated nonseminomatous germ cell tumors often combines surgery and chemotherapy. The goal of initial therapy is never palliation or prolongation of survival, but cure.
Epidemiology
Incidence
Age-related incidence of testicular cancer reveals a bimodal distribution.1 The major peak occurs between ages 15 and 35 years, owing almost exclusively to tumors of germ cell origin, which account for approximately 95% of all testicular cancer. Embryonal carcinoma represents the predominant histopathologic diagnosis up to the age of 35 years, after which seminoma is more common. From 2001 to 2005, the median age at diagnosis for cancer of the testis was 34 years of age.2
The incidence of testicular cancer varies based on geographic distribution. The incidence is highest in northern Europe and North America and lowest in Asia and Africa. There is also a striking influence of race, with the incidence among black and Hispanic males worldwide far less than that for their white counterparts.3, 4 In the United States, estimates of the incidence ratio between white and African-American patients range from 4 to 5 : 1. Testicular cancer appears to be increasing among young white males in the Scandinavian countries, the United Kingdom, and the United States.5 Standardized incidence rates increased annually 2–5%, with marginal differences between seminomas and nonseminomas. In the United States, the annual percentage change from 1989 to 2005 was 0.8%. It is estimated that 8090 cases of testicular cancer were diagnosed in the United States in 2008, with approximately 380 persons dying of the disease.6
Risk factors
Cryptorchidism is the major identifiable risk factor associated with the development of testicular cancer, with a risk ratio reported between 2.5 and 14 in case–control studies.7 The location of the maldescended testicle appears to be an important cofactor, because those patients with intra-abdominal retention have a fourfold higher incidence of malignancy than those with the testicle retained in the inguinal canal. It seems unlikely that maldescent alone represents the initiating event in the development of germ cell tumors (GCTs): Only 10% of testicular tumors are associated with cryptorchidism, whereas 10–20% of the malignancies in patients with cryptorchidism occur in the contralateral, normally descended testicle; prepubertal orchiopexy fails to prevent the subsequent development of malignancy in the undescended testicle; and first-degree male relatives of patients with testicular cancer exhibit an increased incidence of cryptorchidism, hydroceles, and inguinal hernias, as well as testicular cancer.8, 9 These data suggest that some genetic predisposition and/or in utero environmental event may result in several genitourinary developmental abnormalities, including maldescent and germ cell neoplasia. An increase in the frequency of cryptorchidism has been observed and appears to parallel the timing and magnitude of the increase in incidence of testicular cancer. Brothers of men with testicular germ cell tumors (TGCTs) have an 8-fold to 10-fold risk of developing TGCT, whereas the relative risk to fathers and sons is approximately fourfold. This familial relative risk is much higher than that for most other types of cancer. A genome-wide linkage search yielded evidence for a testicular cancer susceptibility gene on chromosome Xq27 that may also predispose to undescended testes.10
TGCTs occur at increased frequency in men with human immunodeficiency virus (HIV). In one multicenter study, 35 patients with HIV-related GCTs were identified. The median age at GCT diagnosis was 34 years (range, 27–64 years). The median CD4 cell count was 315/mm3 (range, 90–960/mm3). There were seminoma in 26 patients (74%) and nonseminomatous germ cell tumor (NSGCT) in nine patients (26%). Twenty-one patients (60%) had stage I disease and 14 patients had metastatic disease. Overall, six patients relapsed, three died from GCT, and seven died from HIV, resulting in a 2-year overall survival rate of 81%. HIV-related seminoma occurred more frequently than in the age-matched and sex-matched HIV-negative population, with a relative risk of 5.4 (95% confidence interval [CI] = 3.35–8.10); however, NSGCT did not occur more frequently, and there was no change in the incidence of GCT since the introduction of highly active antiretroviral therapy. The authors concluded that testicular seminoma occurs significantly more frequently in HIV-positive men than in the control population. Patients with HIV-related GCTs present and should be treated in a similar manner to those in the HIV-negative population. Most of the mortality relates to HIV infection.11
An additional predisposition is the association of mediastinal NSGCTs with Klinefelter’s syndrome. Approximately 10% of all patients with mediastinal nonseminoma have Klinefelter’s syndrome, and there does not appear to be an increased incidence in patients with testicular or retroperitoneal primary tumors.12
An association of dysplastic nevus syndrome and testicular cancer has been observed.13 A twofold higher incidence of multiple atypical nevi, and the attendant risk of melanoma, has been noted.
Patients with a history of unilateral testicular cancer are at risk of developing cancer in the other testicle. In a large series, 2.7% of 2338 patients developed a contralateral testicular tumor during the period of follow-up.14–16 Investigators at the Royal Marsden Hospital reported a similar rate of 2.75% for developing contralateral tumors among 760 men in an interval as long as 15 years.17
Between 1950 and 2001, 3984 patients with testicular cancer were treated at Memorial Sloan Kettering for GCT. A total of 58 patients with bilateral TGCTs were identified. Median follow-up was 60 months. Ten of the 58 patients (17%) had synchronous tumors, while the other 48 (83%) had metachronous tumors. Overall, seminoma was the most common histology of the synchronous and metachronous tumors. Most patients in the synchronous and metachronous tumor groups presented with low-stage disease. Of the 58 patients, 52 (89%) had no evidence of disease, and 6 (11%) were dead of disease at the last follow-up. Treatment of the second tumor appeared to be influenced by therapy for the first tumor in 16.7% of cases.18
Some clinicians recommend routine biopsy of the contralateral testis for patients diagnosed with unilateral testicular cancer. Fossa and colleagues evaluated the risk of contralateral testicular cancer and survival in a large population-based cohort of men diagnosed with testicular cancer before age 55 years using Surveillance, Epidemiology, and End Results (SEER) data. From 29,515 testicular cancer cases reported to the SEER Program of the National Cancer Institute (NCI), from 1973 through 2001, estimates of prevalence of synchronous contralateral testicular cancer, the observed-to-expected ratio (O/E), 15-year cumulative risk of metachronous contralateral testicular cancer, and the 10-year overall survival rate of both synchronous and metachronous contralateral testicular cancer were made. A total of 175 men presented with synchronous contralateral testicular cancer; 287 men developed metachronous contralateral testicular cancer (O/E = 12.4 [95% CI = 11.0–13.9%]; 15-year cumulative risk = 1.9% [95% CI = 1.7–2.1%]). In the multivariable analysis, only nonseminomatous histology of the first testicular cancer was associated with a statistically significantly decreased risk of metachronous contralateral testicular cancer (hazard ratio [HR] = 0.60; 95% CI = 0.46–0.79%; p < 0.001). Increasing age at first testicular cancer diagnosis was associated with decreasing risk of nonseminomatous metachronous contralateral testicular cancer (odds ratio (OR) = 0.90; 95% CI = 0.86–0.94%). The 10-year overall survival rate after metachronous contralateral testicular cancer diagnosis was 93% (95% CI = 88–96%), and that after synchronous contralateral testicular cancer was 85% (95% CI = 78–90%). The low cumulative risk of metachronous contralateral testicular cancer and favorable overall survival of patients diagnosed with metachronous contralateral testicular cancer is in accordance with the current US approach of not performing a biopsy on the contralateral testis.
These observations underscore the importance of continued long-term follow-up of patients with TGCTs.
Pathology
Origin and molecular genetics
Testicular tumors fall into several broad groups (Table 1). Classification of the GCTs has been based on morphology, but recent molecular studies have yielded a more ontological scheme consisting of five distinct subtypes that differ in their proposed cell of origin.19 Accordingly, teratomas and yolk sac tumors arising in neonates and young children derive from primordial germ cells or very early gonocytes distributed along the gonadal ridge or in the testis/ovary. These tumors retain most of the genomic imprinting from both parental genomes. The teratomas remain diploid, while the yolk sac tumors show gains of chromosomes 1q, 12p13–14, and 20q, and losses of 1p, 4, and 6q.19
Table 1 Primary tumors of the testis
| Type | Relative frequency | Genotype/comments |
| Germ cell tumors | ||
| Infants and children | ∼1% of all testis tumors | |
| Yolk sac tumor | 65–80% of prepubertal | Aneuploid |
| Teratoma | 20–35% of prepubertal | Diploid; mature elements; benign |
| Adolescents and adults | 95% of all testis tumors | |
| ITGCNU | >90% of postpubertal | Aneuploid (near triploid) |
| Seminoma | ∼45% of postpubertal | Aneuploid (near triploid); iso12p |
| Nonseminomatous (NSGCT) | ∼55% of postpubertal | Aneuploid (near triploid); iso12p |
| Embryonal carcinoma | ∼75% of NSGCT | |
| Yolk sac tumor | ∼50% of NSGCT | |
| Teratoma | ∼50% of NSGCT | Malignant (even mature elements) |
| Choriocarcinoma | ∼10% of NSGCT | |
| Adults (usually >50 years) | ||
| Spermatocytic seminoma | <1% of postpubertal | Variable ploidy; gain of chromosome 9 |
| Spermatocytic seminoma with sarcoma | Very rare | |
| Sex cord-stromal tumors | ||
| Leydig cell tumor | ∼3% of all testis tumors | 7–10% metastasis (postpubertal) |
| Sertoli cell tumor | <1% of all testis tumors | |
| Granulosa cell tumor | ||
| Adult type | Very rare | |
| Juvenile type | Uncommon | Infants <6 months |
| Mixed/indeterminate | Rare | |
| Mixed germ cell/sex cord-stromal tumors | ||
| Gonadoblastoma | Very rare |
Spermatocytic seminoma is a second, distinct subtype of GCT thought to derive from postpubertal spermatogonia/spermatocytes. Accordingly, these tumors have a paternal pattern of genomic imprinting and show variable ploidy, sometimes with a gain of chromosome.10
Two of the other five proposed subtypes of GCT do not occur in the testis. Dermoid cysts of the ovary, which are thought to arise from oogonia/oocytes, are diploid/tetraploid and show maternal genomic imprinting. Hydatidiform mole (gestational trophoblastic disease) is a placental-derived neoplasm that contains a purely paternal genome as a result of fertilization of an empty ovum.
The fifth subtype of GCT consists of seminoma and the NSGCT patterns that, together, account for 95% of primary testicular neoplasms (Table 1). Variants of this GCT subtype also occur in the ovary (dysgerminoma), anterior mediastinum, and midline brain (germinoma). It is suggested that seminoma and NSGCT are derived from gonocytes that have lost their genomic imprinting as a result of being later in their development than those that give rise to infantile teratomas and yolk sac tumors. These gonocytes are polypoid (triploid or tetraploid), probably because of meiotic arrest. Depending on exactly when this arrest occurs during fetal development, the affected cells may be distributed to one or both testes, accounting for the bilateral GCTs observed in 2–3% of patients.
Seminomas and NSGCT share a common precursor lesion called intratubular germ cell neoplasia, unclassified (ITGCNU). Growing in situ within seminiferous tubules, ITGCNU cells express markers shared with embryonic stem cells, including the transcription factors OCT3/4 and NANOG.20, 21 These factors are essential to the development of embryonic stem cells in mice, but are not expressed in normal spermatogonia in mice or humans. Their presence in ITGCNU supports the theory that a pluripotent gonocyte is the cell of origin for both seminoma and NSGCT. In addition, OCT3/4 serves as a highly specific immunohistochemical marker in the diagnosis of extra-TGCTs (Table 2).20, 22
Table 2 Markers of testicular germ cell tumors
| Morphologic subtype | Serum | Immunohistochemistry | FISH |
| ITGNCU | PLAP, KIT, OCT3/4 | ||
| Seminoma | HCG (low) | PLAP, KIT, OCT3/4 | Excess 12p |
| Embryonal carcinoma | HCG (low) | CD30, OCT3/4, PLAP | Excess 12p |
| Yolk sac tumor | AFP | AFP, PLAP | Excess 12p |
| Choriocarcinoma | HCG (high) | HCG | Excess 12p |
| Teratoma | Excess 12p |
Abbreviations: AFP, α-fetoprotein; FISH, fluorescence in situ hybridization; HCG, human chorionic gonadotropin; PLAP, placental/germ cell alkaline phosphatase.
Progression of ITGCNU to an invasive GCT is accompanied by a number of common events.23 One is the acquisition of excess genetic material from the short arm of chromosome 12. In 80% of cases, this is accomplished through loss of 12q and reduplication of 12p (isochromosome 12p), while in 20%, the additional 12p sequences are distributed among other derivative chromosomes. Interestingly, the embryonic stem cell gene NANOG is on 12p. Fluorescence in situ hybridization (FISH) for 12p is used in paraffin sections as a diagnostic marker for GCTs and also for nongerm cell derivatives.
Additional events associated with malignant progression of ITGCNU include loss of expression of the homeobox gene NKX3.1,24 loss of the tumor suppressor PTEN,25 and decreased expression of the cell cycle regulator p21.26 Mutations of TP53 are rare in postpubertal GCTs, but the effects of this important tumor suppressor may be by MDM2 overexpression 26 or downregulation of LATS2 by microRNAs mi-R372 and mi-R373. A genetic screen implicates miRNA-372 and miRNA-373 as oncogenes in TGCTs.27
Although seminoma and NSGCT share a common origin, they are clinicopathologically distinct cancers. Little is known of what determines their differences, but oncogenic mutations in KIT (a receptor tyrosine kinase) are found in 25% of seminomas and are essentially absent in NSGCT. These mutations may occur very early in seminoma tumorigenesis, as they are present in ITGCNU,28 and in dysgerminoma/germinoma of the ovary, mediastinum, and brain. Based on studies in mice, KIT gene function is essential to the development of primordial germ cells and to normal spermatogenesis; therefore, constitutive activation of this kinase may favor the seminoma pathway. Unfortunately, KIT kinase inhibitors such as imatinib are not likely to be of benefit to seminoma patients harboring KIT-mutant tumors, because most of the published mutations are inherently resistant to the available drugs.
Seminoma
Approximately 45%of all postpubertal TGCTs are pure seminoma (“classic” seminoma). The incidence is increased to 60% in cryptorchid testes. On gross examination, such tumors are generally homogeneous and well demarcated. Distinct lobulation may be apparent, with the nodules separated by dense fibrous bands. Areas of necrosis and hemorrhage are usually discrete. Microscopically, there is a monotonous distribution of uniform, rounded cells with large, centralized nuclei and nucleoli. The cytoplasm may be either clear or granular and will frequently stain for glycogen, lipid, and/or placental/germ cell alkaline phosphatase (PLAP). Stromal elements include an infiltrate rich in T lymphocytes and containing occasional granulomas (Figure 1). These features may mimic granulomatous orchitis.
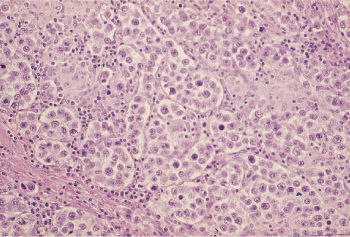
Figure 1 Small nests of seminoma are separated by a lymphoid infiltrate and a focal granulomatous reaction.
Seminoma presents most commonly in the fourth and fifth decades, usually as an enlarging, painless testicular mass. Approximately 70% of patients present with stage I disease, 20% with stage II, and only rarely with disease above the diaphragm. Lymphatic spread is to the para-aortic (PA) lymph nodes and then to the mediastinal or supraclavicular lymph nodes. Hematogenous dissemination to the lung, liver, bone, or adrenal is a late occurrence. Seminomas contain syncytiotrophoblastic giant cells that stain for human chorionic gonadotropin (HCG). Low-level HCG elevation is seen in 5–10% of patients with pure seminoma and likely reflects syncytiotrophoblastic elements present within the tumor (Table 2). Seminoma does not secrete α-fetoprotein (AFP).
Nonseminomatous germ cell tumors
The most common postpubertal GCTs of the testis are composed of one or more elements that are collectively known as “nonseminomatous.” Four morphologic patterns are recognized among this group. In most cases, these patterns are intermixed in varying proportions, often subtly merging from one to the next. Areas of seminoma may be included (termed “mixed GCTs” by some), but the prognosis is determined by the presence of the other elements and is less favorable, overall, than for pure seminomas.
Embryonal carcinoma
Embryonal carcinoma is present in up to 90% of NSGCT cases. Macroscopically, it forms a soft, fleshy, inhomogeneous mass with areas of necrosis and hemorrhage. Direct invasion of the spermatic cord, epididymis, and tunica albuginea occurs.
The microscopic appearance is extremely variable and may include papillary, solid, tubular, and glandular patterns, frequently interrupted by geographic necrosis (Figure 2). Large polygonal cells with indistinct cytoplasmic borders (unlike seminoma) are the rule, with pale granular cytoplasm, large nuclei, and one or more centrally placed nucleoli. Mitotic figures and multinucleated cells are common. Clinically, these are aggressive tumors with lymph node metastases present in two-thirds of patients.
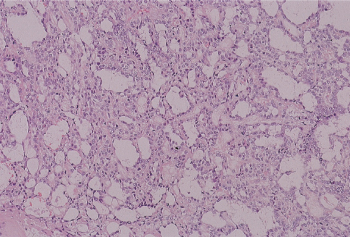
Figure 2 Yolk sac tumor. Numerous microcysts occur in the most common pattern of yolk sac tumor.
Yolk sac tumor
Yolk sac tumor, formerly called endodermal sinus tumor, is present in approximately half of NSGCT cases, but is rare in pure form in the postpubertal patient.29 The most readily recognized pattern consists of a cluster of tumor cells surrounding a small central blood vessel (Schiller–Duval body) (Figure 3). The morphologic spectrum is broad, including microcystic (lacelike), micropapillary, solid, and hepatoid patterns. The tumor cell nuclei are smaller than those of embryonal carcinoma. Cytoplasmic globules are common and stain for AFP, which accounts for the serum elevations characteristically present in patients with this tumor (Table 2).
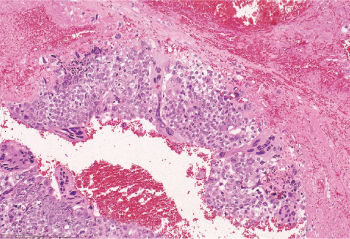
Figure 3 Choriocarcinoma. Syncytiotrophoblastic cells “cap” islands of mononucleated cytotrophoblast. Note the hemorrhagic background.
In its pure form, yolk sac tumor is the most common testicular neoplasm in infants and young children (Table 1). Despite morphologic similarity to the subtype observed in postpubertal NSGCT, the pediatric tumor is an oncogenetically distinct entity and carries a better prognosis.
Choriocarcinoma
Choriocarcinoma is an uncommon element in NSGCT (15%) and is rare as a pure tumor. On gross examination, areas of choriocarcinoma are characteristically hemorrhagic. Microscopically, the diagnosis requires a combination of cytotrophoblasts and syncytiotrophoblasts (Figure 4). Stroma is sparse but tends to be highly vascular. Choriocarcinoma of the testis represents the most aggressive subtype of NSGCT, often presenting with large-volume visceral metastases and/or brain metastases. Extreme elevations of serum HCG levels are characteristic.
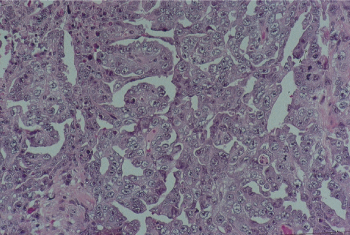
Figure 4 Embryonal carcinoma. Irregularly shaped glands and papillae are lined by pleomorphic cells with vesicular, crowded nuclei and poorly defined cytoplasmic membranes.
Teratoma
Teratoma is defined as a tumor that contains elements of all three germ layers present with varying degrees of differentiation. Teratomatous elements are recognized in approximately half of NSGCT cases, but are not usually pure. Macroscopically, teratomas tend to be large and have multiloculated cysts containing serosanguineous fluid as well as cartilaginous solid areas. Microscopically, all manner of tissue elements may be present, including cysts with squamous, respiratory, or intestinal-type linings, mature cartilage, muscle, and fibroblastic stroma (Figure 5). Areas that are less well differentiated (“immature teratoma”) are often intermixed (Figure 6). Regardless of the degree of differentiation, all teratomas in the postpubertal setting are regarded as malignant. In postchemotherapy specimens teratoma is the most common residual element. The presence of nonteratomatous NSGCT may be an indication for additional therapy.
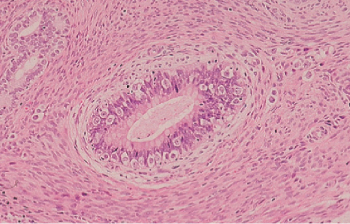
Figure 5 Mature teratoma. There are mature-appearing small glands, a portion of a pilosebaceous unit, and bundles of smooth muscle.
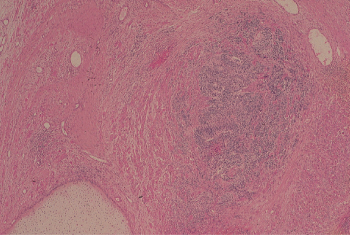
Figure 6 Immature teratoma. An island of immature neuroepithelium is present adjacent to a nodule of hyaline cartilage.
A pure form of mature teratoma is common among pediatric patients under the age of 4 years. Although morphologically similar to mature areas of teratoma within NSGCT, these lesions arise through a different pathway and are essentially benign30 (Table 1). Rarely, a nonteratomatous element is identified and may give rise to metastases.
Nongerm cell cancers arising from germ cell tumors
Given the pluripotent nature of the gonocytes from which seminomas and NSGCT are thought to arise, a nongerm cell element may emerge and become the dominant pattern in advanced cases of postpubertal testicular cancer. Among these are cancers morphologically resembling embryonal rhabdomyosarcoma, adenosquamous carcinoma, leiomyosarcoma, Wilms’ tumor, glioblastoma multiforme, and primitive neuroectodermal tumor (PNET), all of which are associated with resistance to chemotherapy.31 Myelodysplasia and leukemia may also evolve from NSGCT.32
Spermatocytic seminoma
Spermatocytic seminoma accounts for 1–2% of TGCTs. On gross examination, it has a grayish appearance and tends to be softer than classic seminoma. Microscopically, these tumors tend to form tubular clusters and are composed of round cells of highly variable size that bear resemblance to the cellular stages of normal spermatogenesis (Figure 7). In contrast to classic seminoma, stromal lymphocytic infiltration is not a feature of spermatocytic seminoma. This tumor tends to occur over the age of 50, with a median age of 65 years. The prognosis following surgery is excellent, as there are only anecdotal reports of metastases.
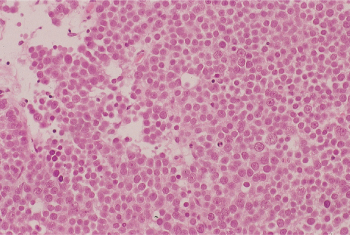
Figure 7 Spermatocytic seminoma. There is a diffuse sheetlike arrangement of neoplastic cells that vary in size.
Sex cord-stromal tumors
Tumors arising from stromal tissue account for only 4% of all adult testicular tumors but represent almost 20% of childhood testicular tumors. These tumors are thought to arise from primitive gonadal mesenchyme and are subcategorized as Leydig cell tumor, Sertoli cell tumor, gonadoblastoma, granulosa cell tumor, and mixed/indeterminate types (Table 1).
Leydig cell tumor
Leydig cell tumor represents about 3% of all testicular tumors, and although they may be seen in children, the median age of appearance is 60 years. Histologically, they are typified by cells with abundant oncocytic cytoplasm and round, regular nuclei. Clinical symptoms are usually related to the production of both androgens and estrogens by tumor cells, leading to precocious puberty in a child and gynecomastia in the adult. Approximately 10% of Leydig cell tumors metastasize, but this occurs only in the postpubertal patient. In patients with metastatic disease, treatment with radiation or chemotherapeutic agents has generally been ineffective. Retroperitoneal lymph node dissection (RPLND) is an important staging procedure. The therapeutic role of RPLND in low-volume metastatic retroperitoneal disease is unclear, but waiting to resect higher-volume tumor is ineffective. A prophylactic RPLND should, therefore, be considered in patients with clinical stage I tumors.
Sertoli cell tumor
Sertoli cell tumor shows no age predilection, presenting as a testicular mass that may be accompanied by gynecomastia or impotence secondary to estrogen production. Microscopically, these lesions are composed of rounded cells growing in cords and sheets in a fibrous background. Therapy is primarily directed at resection of the primary lesion, with a staging work-up to include abdominal computed tomography (CT) scan and chest radiography. RPLND is controversial in clinical stage I disease. Large primary tumors with frequent mitoses or necrosis may prompt an RPLND. Sertoli cell tumors can respond to platinum combination chemotherapy.
Clinical presentation
Most patients seek medical attention because of a swollen testis. Accompanying symptoms include a sensation of heaviness or aching in the affected gonad. Severe pain is rare, unless there is associated epididymitis or bleeding in the tumor. Because testicular cancer is commonly associated with low sperm counts, patients may present during an infertility work-up.
Approximately 25% of patients with disseminated disease present with symptoms from metastatic disease.33 Severe back pain from metastasis to the retroperitoneum is the most frequent and is the presenting symptom in patients with primary retroperitoneal GCTs. Shortness of breath, chest pain, and hemoptysis are usually manifestations of advanced lung metastases. Primary mediastinal GCTs are an exception, in that these tumors (if malignant) present with symptoms of mediastinal compression with pain, dysphagia, shortness of breath, and superior vena cava syndrome. Teratomas of the mediastinum produce few symptoms and are commonly discovered on routine chest film obtained for minor chest complaints.
Diagnosis
Understanding the diagnosis and staging of TGCTs depends on understanding the anatomy of the vascular and lymphatic drainage of the testis as well as the likely sites of metastatic spread of the disease. The spermatic cord contains the lymphatic and vascular supply of the testis. The lymphatic and vascular supply diverges medially when the spermatic vessels cross ventral to the ureter. The landing zones for the lymphatic drainage of the right testis are the interaortocaval nodes below the renal vasculature and the ipsilateral distribution of nodes, especially the paracaval and preaortic nodes. The primary landing zone for a left-sided primary tumor includes in the PA nodes below the left renal vessels and the PA and preaortic nodes. Ipsilateral common iliac nodes are uncommonly involved unless large-volume disease is present.
Unusual patterns of disease can be seen (or created) in patients who have had prior pelvic surgeries including herniorrhaphy, abdominal orchiopexy, or scrotal violations.34 The proper and only diagnostic procedure in this setting is a radical inguinal orchiectomy. Transscrotal procedures can disrupt predictable patterns of lymphatic metastases and should not be done.
Tumors of the testis can present with a discrete nodular density or as diffuse infiltration of the entire testis (particularly seminoma and lymphoma). The other testis serves as a useful reference standard. If a testicular mass is suspected, transscrotal ultrasonography should be performed. The presence of a hypoechoic mass represents a testicular neoplasm, and a radical inguinal orchiectomy is required to make a diagnosis and to ensure local control of a primary testicular cancer.
Extragonadal germ cell tumors (EGCTs) arising within the retroperitoneum or mediastinum require specialized management. A diagnosis may be made on the basis of significantly elevated tumor markers in a patient with a mass in the anterior mediastinum or retroperitoneum. If there is no marker elevation, tissue confirmation is required. Chemotherapy is the primary treatment; attempts at debulking or total removal of mediastinal GCTs as initial management are inappropriate. Primary retroperitoneal GCTs may be associated with an occult testicular primary. Such patients should have a thorough evaluation of the gonads, including the use of testicular ultrasonography. If a previously unsuspected testicular tumor is found, orchiectomy can serve as the diagnostic procedure. Otherwise, fine-needle aspiration of the abdominal mass or exploratory laparotomy is required. In addition, an i(12p) chromosomal abnormality is diagnostic for GCT in a patient who presents with undifferentiated cancer.
A transscrotal biopsy should never be performed. If, at the time of scrotal orchiectomy, the surgeon identified the tumor and removed the testis in toto, then the inguinal portion of the spermatic cord must be removed. If a testicular biopsy was performed, management of the hemiscrotum depends on the primary treatment modality. Patients who are receiving primary chemotherapy do not need hemiscrotectomy. Inguinal lymphadenectomy is reserved for patients with palpable inguinal lymphadenopathy. For patients with early-stage seminoma who have had a scrotal violation, approximately 5–10% will experience local failure. Extending the field to include the groin and scrotum diminishes these prospects but is associated with increased infertility.
Tumor markers
Serum HCG and AFP have significant value in the diagnosis, prognosis, and management of patients with GCTs. AFP is derived from the yolk sac or embryonal carcinoma elements of germ cell cancers. Elevation of AFP is not seen in normal adults. The half-life of this protein in the serum is approximately 5 days. In germ cell cancers, syncytiotrophoblastic components elaborate HCG. The protein comprises an alpha subunit and a beta subunit, each of which is antigenically distinct. The serum half-life of the entire protein is 18–30 h.
Serum HCG and AFP are elevated in 85% of patients with disseminated NSGCTs. AFP alone is elevated in 40% of patients, and HCG alone is elevated in 50–60% of patients with disseminated nonseminomatous testicular cancer. Elevated lactic acid dehydrogenase (LDH) is less specific and is mainly a correlate of disease bulk.
Pure seminoma is most frequently associated with normal AFP and HCG, but approximately 10% of all cases, and up to 30% of patients with advanced disease, may have low levels (usually <100 mIU/mL).35 Any elevation of AFP in patients with seminoma must be viewed as evidence of nonseminomatous disease, and management should proceed accordingly.
AFP and HCG should be determined before and after orchiectomy, but the absence of marker elevation should not influence the decision to undertake the procedure. Likewise, normalization of serum markers after orchiectomy does not ensure that all disease has been removed, although persistence of marker elevation implies residual disease.
The rate of disappearance of elevated tumor markers is very useful in determining response to chemotherapy. HCG is the most useful in this regard; a 10-fold decrease in the HCG level over a 3-week period is consistent with potentially curative chemotherapy. Less steep declines of HCG levels may correlate with the emergence of drug resistance. Likewise, the reappearance of markers often predates the radiographic appearance of recurrent disease.
The presence of tumor markers also can lead to errors in clinical management. First, HCG determination can be nonspecific, and there is some cross-reactivity in the radioimmunoassay with luteinizing hormone. Also, HCG can be falsely elevated in patients who use marijuana. HCG determination should be repeated to ensure that the elevation is not a laboratory error. If the level is still high, the patient should be queried regarding drug usage. Testosterone should be given in a dose of 300 mg intramuscularly to ensure that a hypogonadal state with resultant high levels of luteinizing hormone is not interfering with the determination of HCG. If the level remains increased, restaging procedures and investigation of sanctuary sites (brain and contralateral testis) are in order. A retrospective review by Zon and colleagues evaluated management problems in patients with very high HCG levels.36 Forty-one patients with an HCG >50,000 mIU/mL were included. All patients received cisplatin-based chemotherapy. Two of these 41 patients had normal HCG levels at the time of the fourth course of chemotherapy. Eight additional patients had normalized the HCG within 1 month of completion of the fourth course of therapy. Of these ten patients, seven remain continuously free of disease, and three are currently disease-free with salvage therapy. Thirty-one patients still had an abnormal HCG more than 1 month after completing the fourth course of primary chemotherapy. Fifteen of these patients are continuously disease-free, despite no further treatment. A subset of patients with very high HCG levels do not have a consistent predictable decline of HCG with treatment. Absolute dependence on predicted patterns of decline in these patients would have resulted in overtreatment. Our strategy has been to wait for a rising serum HCG before considering salvage therapy.
False-positive elevation of AFP is quite rare. Differential considerations include laboratory error, other tumor types (such as hepatocellular carcinoma), and liver inflammation from cirrhosis or hepatitis. An occasional patient may have baseline elevation of AFP (usually <100 ng/mL) that remains static over time and does not reflect active disease. Some individuals have familial, hereditary, mildly elevated serum AFP levels in the range of 15–30 ng/mL. Patients with clinical stage I disease, normal imaging, normal contralateral testicle, and a minimally elevated AFP level (<25 ng/mL) should be observed and only be treated if there is a clear AFP increase and/or development of metastases.37
Staging
GCTs are typically categorized as stage I, referring to tumors confined to the testis; stage II, indicating metastatic disease to the nodes of the periaortic or vena caval zone without pulmonary or visceral involvement; and stage III, which denotes metastasis above the diaphragm or involving other viscera. The AJCC and World Health Organization (WHO) classification is the international standard classification (Table 3).39
Table 3 AJCC staging
| TNM clinical classification | |
| T: | Primary tumor: The extent of the primary tumor is classified after radical orchiectomy (see pT). If no radical orchiectomy has been performed, TX is used |
| N: | Regional lymph nodes |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis with a lymph node mass ≤2 cm in greatest dimension or multiple lymph nodes, not >2 cm in greatest dimension |
| N2 | Metastasis with a lymph node mass >2 cm but not >5 cm in greatest dimension, or multiple lymph nodes, any one mass >2 cm but not >5 cm in greatest dimension |
| N3 | Metastasis with a lymph node mass >5 cm in greatest dimension |
| M: | Distant metastasis |
| MX | Distant metastasis cannot be assessed |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
| M1a | Nonregional lymph node or pulmonary metastasis |
| M1b | Distant metastasis other than to nonregional lymph node and lungs |
| pTNM pathologic classification | |
| pT: | Primary tumor |
| pTX | Primary tumor cannot be assessed (if no radical orchiectomy has been performed, TX is used) |
| pT0 | No evidence of primary tumor (e.g., histologic scar in testis) |
| pTis | Intratubular germ cell neoplasia (carcinoma in situ) |
| pT1 | Tumor limited to testis and epididymis without vascular/lymphatic invasion; tumor may invade tunica albuginea, but not tunica vaginalis |
| pT2 | Tumor limited to testis and epididymis with vascular/lymphatic invasion, or tumor extending through tunica albuginea with involvement of tunica vaginalis |
| pT3 | Tumor invades spermatic cord with or without vascular/lymphatic invasion |
| pT4 | Tumor invades scrotum with or without vascular/lymphatic invasion |
| pN: | Regional lymph nodes |
| pNX | Regional lymph nodes cannot be assessed |
| pN0 | No regional lymph node metastasis |
| pN1 | Metastasis with a lymph node mass ≤2 cm in greatest dimension and ≤5 positive nodes, none >2 cm in greatest dimension |
| pN2 | Metastasis with a lymph node mass >2 cm but not >5 cm in greatest dimension; or >5 nodes positive, not >5 cm; or evidence of extranodal extension of tumor |
| pN3 | Metastasis with a lymph node mass >5 cm in greatest dimension |
| pM: | Distant metastasis |
| The pM category corresponds to the M category | |
| S: | Serum tumor markers |
| SX | Serum marker studies not available or not performed |
| S0 | Serum marker study levels within normal limits LDH, HCG, and α-fetoprotein |
| S1 | <1.5.N and <5000 and 1000 |
| S2 | 1.5–10.N or 5000–50,000 or 1000–10,000 |
| S3 | >10.N or >50,000 or >10,000 |
N, the upper limit of normal for the lactate dehydrogenase assay.
Source: Edge 2010.38 Reproduced with permission of Springer.
Standard procedures to establish clinical stage include physical examination, abdominal and chest CT scans, and serum levels of AFP, βHCG, and LDH. Brain imaging and bone scans should be performed only when clinically indicated (e.g., clinical symptoms and/or poor-risk disease). The role of positron emission tomography (PET) remains investigational in the initial staging of patients with GCTs and is not considered a standard staging procedure. PET scan will not reliably detect teratoma or microscopic carcinoma. PET scan has been investigated in patients with residual masses after chemotherapy for seminoma, and the persistence of PET avidity is associated with risk of recurrence, but a PET scan cannot differentiate between residual teratoma and necrosis.40
Therapy
Carcinoma in situ
Carcinoma in situ (CIS) or tubular intraepithelial neoplasia (TIN) is a true premalignant condition leading to both seminomatous and nonseminomatous invasive GCTs in up to 50% of untreated cases (Figures 8 and 9).41 Management is controversial. Observation after diagnosis of TIN offers the best chance of retained fertility but requires a compliant patient and risks the possible requirement for more intensive treatment of invasive disease. Low-dose radiotherapy (18–20 Gy in 1.5–2.0 Gy fractions) will eradicate the TIN with high probability of decreased or eliminated fertility without affecting potency.41, 42 Total orchiectomy will obviously eliminate TIN in the effected testicle, as well as all germ cells and Leydig cells within that testicle. It may be the favored option in some patients with TIN in one testicle and a normal contralateral testicle. Partial orchiectomy alone is ill advised because TIN is typically a diffuse process within the effected testicle. Chemotherapy is not indicated for TIN.43
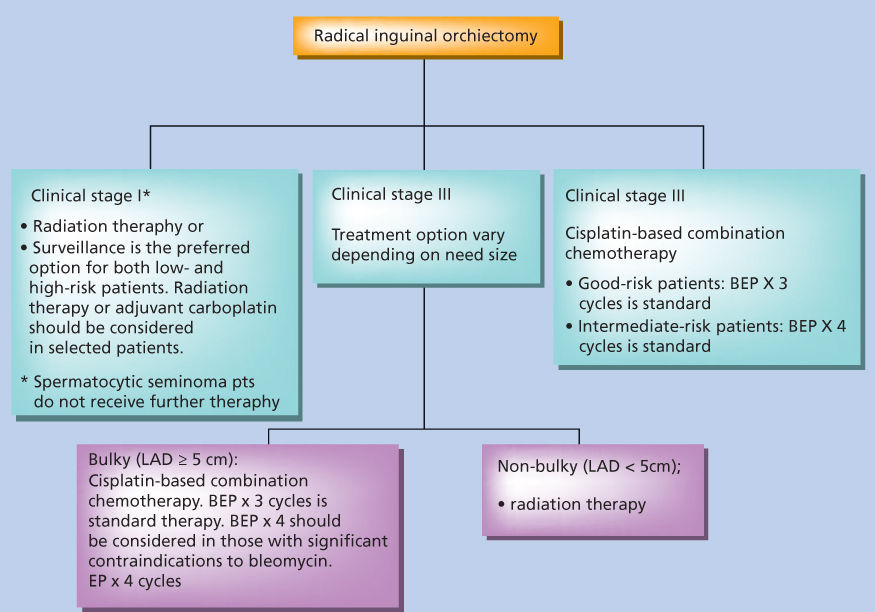
Figure 8 Treatment of seminoma.
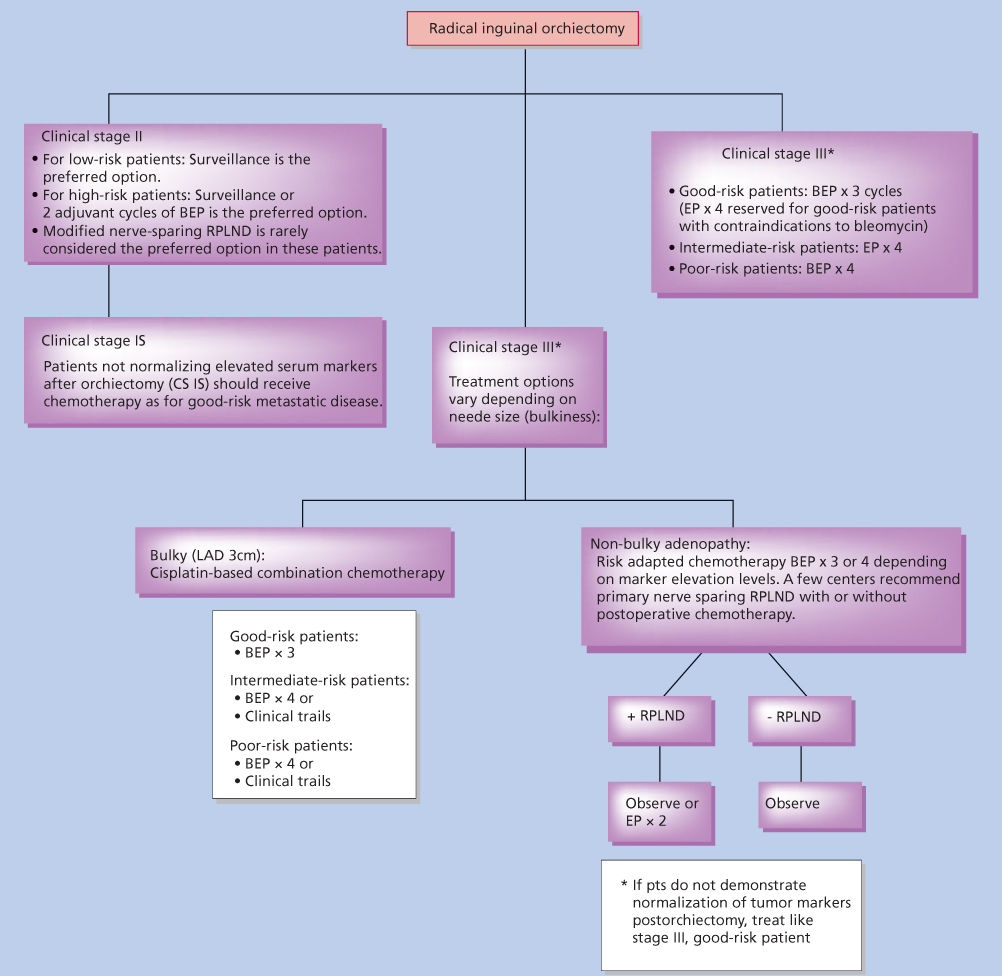
Figure 9 Treatment of nonseminoma.
Nonseminoma: Early stage
Knowledge of the natural history of testicular primary lesions and their lymphatic drainage patterns is key to understanding the therapeutic options. Testicular lymphatics arise in proximity to the embryonic origin of the testicle, in the genital ridge in the high lumbar region. Although the afferent lymphatic channels accompany testicular descent into the scrotum, draining lymph nodes remain in the retroperitoneum.
In 1910, Jamieson and Dobson demonstrated that the drainage pattern for testis tumors differed according to the side of the primary lesion, with right-sided lesions draining to the paracaval, interaortocaval, and preaortic nodes and left-sided lesions to the PA and preaortic nodes.44
Stage I nonseminoma
The cure for patients with stage I nonseminoma is close to 100%. Three therapeutic options exist—primary RPLND, adjuvant chemotherapy, and active surveillance—all of which result in a similarly excellent outcome. Attention has therefore been focused on the reduction in toxicity. The accurate diagnosis of clinical stage I disease is critical. Following inguinal orchiectomy and the diagnosis of nonseminoma, serum levels of β-HCG and AFP must return to normal (if elevated before orchiectomy), and abdominal CT, chest radiography, and/or chest CT must all be negative before a patient can be labeled as having clinical stage I disease. In this clinical setting, approximately 30% of patients will suffer a relapse if no other therapy is administered, with the retroperitoneum remaining as the area at highest risk. Approximately 8–10% of patients will develop metastases outside of the retroperitoneum, mostly in the lungs.45
Freedman and colleagues developed a mathematical model on the basis of the four identified prognostic factors and were able to identify a subset of patients with a 58% relapse rate at 2 years.46 These four factors included invasion of testicular veins, invasion of testicular lymphatics, absence of yolk sac elements, and presence of undifferentiated tumor. Pathologic T-stage higher than 1, the presence of components of embryonal carcinoma, and percent of the primary tumor occupied by teratoma have also been described.46–48 The importance of vascular invasion as the most dominating independent risk factor has been emphasized. Clinically, the presence of vascular invasion in the primary tumor specimen discriminates the “high-risk” patients with a risk of relapse of approximately 50% from the “low-risk” patients without lymphovascular invasion and an approximately 15–20% risk of relapse.
There is an ongoing controversy regarding the optimal management of patients with clinical stage I nonseminoma. All three options—RPLND, adjuvant chemotherapy, and active surveillance—are discussed later.
Retroperitoneal lymph node dissection
Based on a predictable lymphatic pattern of spread of testicular tumors, RPLND emerged as a treatment option for testicular cancer as early as 1907.49–51 In the United States, primary RPLND became the conventional approach for patients with clinical stage I NSGCTs. In Europe and Canada, consensus guidelines do not currently advocate primary RPLND in early-stage disease.52, 53 Nevertheless, RPLND does provide the most accurate method of detecting retroperitoneal nodal disease, which account for more than 90% of the first site of metastatic spread. RPLND alone can be curative in 50–80% of clinical stage I disease who are found to have limited nodal disease at surgery (pN1). More than five metastatic lymph nodes, diseased nodes measuring more than 2 cm, and any extranodal extension (pN2–pN23) are virtually all cured following two cycles of adjuvant chemotherapy.54 Primary RPLND also has the advantage of removing retroperitoneal teratoma, which is chemoresistant, virtually eliminating the risk of late relapse of teratoma.55 However even in pure teratomas, only 20% of patients will harbor occult retroperitoneal lymph node metastases at the time of diagnosis.56 Relapses in the retroperitoneum are exceedingly rare in patients who undergo RPLND by experienced surgeons. The overall relapse rate for patients with disease limited to the testicle is approximately 10%, with the great majority of relapses occurring in the lungs.
Radical lymphadenectomy via a thoracoabdominal (extraperitoneal) approach was described in 1950 57 and popularized by Skinner in the 1980s,58 while reports of pure abdominal (anterior) approaches dominated the 1970s,59, 60 with each approach having intrinsic advantages. The thoracoabdominal approach is associated with significantly higher morbidity including pain and chest complications albeit lower rates of small bowel obstruction.58 To reduce morbidity Donohue and colleagues described the midline anterior approach, and since the incidence of suprahilar metastases in clinical stage I disease was shown to be rare, the technique evolved to include only infrahilar RPLND.61 The thoracoabdominal incision has largely been replaced by the anterior midline approach.
In experienced hands, the classic bilateral RPLND is associated with minimal perioperative morbidity and virtually no mortality.62 A major side effect is the loss of antegrade ejaculation, with resultant need for assisted reproductive technology in over 90% of patients. An improved understanding of the nerves and pathways responsible for seminal emission and ejaculation along with meticulous anatomic studies of the distribution of right-sided and left-sided tumors led to further modification of the surgical “templates” for RPLND.63, 64 Several nerve-sparing modifications of the classic node dissection have now been described.65–67 Any template used must adhere to strict principles of thoroughly resecting all interaortocaval lymph nodes and ipsilateral lymph nodes from the level of the renal hilum down to the bifurcation of the ipsilateral common iliac artery. Dissection is minimized on the contralateral side particularly below the level of the inferior mesenteric artery (IMA). To minimize retroperitoneal recurrence, some advocate bilateral infrahilar dissections only sparing the contralateral nodes below the IMA.68 Modified template nerve-sparing RPLND is now considered standard for patients undergoing surgery for clinical stage I disease. Meticulous preservation of the postganglionic sympathetic fibers arising from the sympathetic chain and the hypogastric plexus results in uniformly high rates of preservation of ejaculatory function (96–100%) while maintaining more than 99% cure rate.59, 66, 69
To further reduce the morbidity of surgery, laparoscopic techniques for RPLND have been used.70 Although operating room times are longer, long-term follow-up in over 550 patients has shown no difference in relapse rates compared with traditional open RPLND. However, the vast majority (>90%) of the patients with positive nodes have received adjuvant chemotherapy raising the question of the true efficacy of this approach.
Radiotherapy
Although previously utilized in stage I nonseminoma, radiotherapy is no longer used based on the overwhelming success of combination chemotherapy, the safety of active surveillance, as well as the limited efficacy of radiotherapy in nonseminomas.
Adjuvant chemotherapy
The definition of a high-risk group by vascular invasion, the efficacy and safety of chemotherapy for good-risk metastatic disease, and the near-perfect results of two cycles of chemotherapy in the setting of fully resected stage II disease have prompted investigators to consider the use of primary chemotherapy in high-risk stage I disease.
Based on data from stage II trials suggesting that two cycles of bleomycin, etoposide, and cisplatin (BEP) may be sufficient adjuvant treatment, the MRC designed a prospective study offering two cycles of BEP to patients with high-risk stage 1 NSGCTT71 to evaluate the efficacy and long-term toxicity of adjuvant chemotherapy. One hundred and fourteen patients were treated and followed up for a median of 4 years. The 2-year recurrence-free survival was 98%. The 95% CI excluded a true recurrence rate of more than 5%. Of the two patients who recurred, one was found to have adenocarcinoma of the rete testis rather than a germ cell tumor. No major clinically significant long-term toxicities were observed, although the median follow-up was only 4 years. Adjuvant chemotherapy with two cycles of BEP achieves a near-universal cure in patients with stage I disease with relapse rates in various studies ranging from 0% to 2%.
Two cycles of adjuvant BEP were subsequently adopted as the standard approach to patients with vascular invasion for the European consensus guidelines, whereas patients with low-risk disease are candidates for active surveillance.52
Recent data suggest that 1 cycle of BEP results in similar outcome and 1 cycle of BEP is now recommended as the adjuvant chemotherapy of choice.72, 73
Active surveillance
The main rationale for active surveillance is that systemic chemotherapy is highly effective and thus patients who are cured by orchiectomy alone can be spared the treatment-related toxicity of a primary RPLND or adjuvant chemotherapy.
An early large prospective study of surveillance included 373 patients with a median follow-up of 5 years.74 The recurrence rate was 27% and of these 80% recurred within the first year. Overall cure rate for the entire cohort of patients exceeded 98%. Vascular invasion was confirmed as the most important prognostic factor. A large data set including 1139 stage I nonseminoma patients recently confirmed active surveillance as an excellent and safe management modality. With a recurrence rate of 19% (44% in lymphovascular invasion-positive patients and 14% in negative patients) and a disease-specific survival of 99.7% on long-term follow-up, this series of surveillance underlines the efficacy of the approach.75
There are now reports of more than 3000 patients worldwide with clinical stage I disease who have had no other therapy administered following orchiectomy with relapse rates and survival remarkably similar between trials, regardless of the size of the study or country of origin.76 Almost all patients on active surveillance relapse within 2 years after diagnosis with IGCCCG good-prognosis disease.74, 77 Only 2–3% of relapses occur beyond 2 years.75 Thus careful surveillance plus chemotherapy at the earliest sign of recurrence is an effective management approach to patients with stage 1 NSGCTT. Active surveillance has been adopted as the standard of care for patients with low-risk disease by the European consensus guidelines and for patients with both low-risk and high-risk disease by the Canadian consensus guidelines.52
Based on a randomized prospective trial, a sufficient follow-up schedule for vascular invasion-negative patients consists of tumor markers and clinical examination every 2 months for the first 2 years and every 4–6 months for years 3–5. Chest X-rays are done every 4 months for the first 2 years and then every 6 months thereafter until year 5.78 CT scans are performed at 3, 12, 24, and 56 months. Various surveillance schedules with closer follow-up and more frequent imaging exist for high-risk patients.
Management preferences in stage 1 NSGCTT
Patients with stage 1 NSGCTT, especially those at high risk of recurrence, have a choice of management options. All options, when carried out meticulously, result in the same excellent survival prospects but with different shortcomings.
Arguments for retaining primary surgery are that when done in one of the few high-volume centers in the United States or elsewhere, results are excellent, infertility and complication rates are very low, and essentially such procedure eliminates the abdomen as a source of relapse. However, even in excellent centers, preoperative evaluations routinely fail to reliably identify the seventy percent of patients who are pathological stage I, thus subjecting the majority of patients to major surgery without therapeutic benefit. In addition, 25–35% of patients will require additional two cycles of BEP due to extensive retroperitoneal disease. Most importantly, RPLND does not eliminate the risk of recurrence outside the retroperitoneum (8–10%). The results of community-level primary surgical management of stage I nonseminoma have demonstrated not only a numerically greater relapse rate as compared to adjuvant chemotherapy but also an increased number of patients experiencing both scrotal and abdominal relapses, strongly suggesting that primary RPLNDs performed by less experienced urologists result in inadequate cancer operations.72
Adjuvant chemotherapy, in particular for high-risk disease, is considered the standard of care in many countries. While the recurrence rate is decreased to 2–4%, adjuvant chemotherapy will also result in overtreatment in at least 50% of patients.76 All of these patients will experience hair loss; a significant disruption from work, school, and life; exposure to significant neutropenia with the rare risk of fatal complications; risk of vascular complications; rare acute chemotherapy reactions; at least temporary effects on fertility; and the anxiety and fears that all patients receiving chemotherapy face. The potential long-term complications are currently unknown and relapses can still occur. Thus, patients treated with adjuvant chemotherapy are not fully spared the fear of relapse or the inconvenience of ongoing imaging. Expert groups are now recommending one cycle of adjuvant BEP for high-risk stage I nonseminoma.73, 79
Concerns regarding the lack of compliance are an argument against surveillance, in particular in high-risk CSI nonseminoma.76 There is no evidence that the level of compliance across varying geographies materially impacts survival.76, 80, 81 Survival rates consistently approach 100% even in series with reported “unsatisfactory” compliance. Educating patients is crucial and emphasizing that later identification of disease might well lead to more complicated and complex therapies is fully warranted. Concerns about the undissected retroperitoneum leading to a significant number of late refractory cancer or late recurrence of teratoma have not been realized in this and other studies.76, 80, 82, 83
On surveillance, only patients who relapse will receive treatment. Treatment of these patients will be slightly longer than adjuvant BEP (6 vs. 9 weeks).84 In our opinion, there is only a small difference in toxicity between two and three courses of chemotherapy. However, there is a major difference between no chemotherapy and two courses. Active surveillance completely spares 70–75% of patients the burden of any active treatment.
Clinical stage II
Tumor marker-positive disease and/or large-volume abdominal disease (>2 cm on abdominal CT, stage IIB) should be treated with primary chemotherapy. Standard treatment is three cycles of BEP for IGCCCG good-prognosis disease.84 Only in the case of significant contraindication to bleomycin four cycles of etoposide and cisplatin (EP) can be considered.85 Most patients with stage IIA/B will achieve a clinical complete remission with resolution of all lymph node metastases and normalization of tumor markers (80–90% in stage IIA and 65–85% in stage IIB).84, 85 Only patients with persistent retroperitoneal residual disease on the postchemotherapy CT scan should undergo RPLND. The relapse rate with this strategy is 4–9% for stage IIA and 11–15% for stage IIB.86–88 Tumor marker-negative stage IIA (lymph nodes ≤2 cm) represents a particular problem. Some of these patients will have benign lymph node enlargement; however, some will have teratoma, pure embryonal carcinoma, or mixed tumors. There is currently no diagnostic tool to determine the nature of these masses reliably. Management options include primary RPLND or a surveillance period with repeat imaging after 6–8 weeks. If the lesions shrink, no further therapy is required; for stable or growing lesions, most centers would consider primary chemotherapy, with a few selected centers considering primary RPLND.
Seminoma: Early stage
Orchiectomy and postoperative radiation therapy constituted the standard of care for early-stage seminoma patients during most of the twentieth century.89–91 A radical orchiectomy by an inguinal approach is highly effective therapy for controlling disease at the primary tumor site. Occult or gross PA lymphatic tumor deposits can be eradicated with high probability after low doses of radiation therapy (20–25 Gy), owing to the extreme radiosensitivity of seminoma. Trials have investigated surveillance in stage I seminoma. With a large majority of patients experiencing prolonged disease-free survival in early-stage seminoma, long-term quality of life will assume, increasing significance in evaluating management options.
Stage I seminoma: Primary and adjuvant therapy
Stage I disease comprises 85% of all seminoma cases.92 Surveillance studies demonstrated that an orchiectomy is curative approximately 80–85% of the time. Chemotherapy and/or radiation can be given as salvage therapy or as prophylaxis. Regardless of the elected management method, disease-specific survival at 5 years is over 99%.93–96 The treatment of relapsed treatment-naïve disease appears to be as curable as it is at first presentation.
Four major series demonstrated that surveillance is a viable option.93–95, 97–100 Their combined experience was pooled to better identify candidates for treatment or surveillance.94 In the entire group of 638 patients, six patients (0.9%) died of disease or complications of treatment of relapse. The overall 5-year and 10-year relapse-free rates were 82.3% and 78.7%, respectively. The majority (68.6%) of relapses occurred within 2 years, but still 7% of recurrences occur after 6 years. The two most important prognostic factors were tumor size and rete testis invasion. Patients with tumors >4 cm in diameter were twice as likely to relapse as those with smaller tumors. The rete testis is a communicating network of seminal channels traversing the mediastinum (or hilum) of the testis. Rete testis invasion was seen in 37% and defined as extension of the tumor into the testicular mediastinum without necessarily involving the tubular lumens. For patients with large tumor size and rete testis invasion, the rates of recurrence were as high as 33%. For patients without these factors, the recurrence rates were as low as 13%. This analysis could not be validated in a subsequent, recently published analysis from the two groups using an independent data set, although tumor size as a linear variable is associated with risk.101, 102
The schedule for surveillance testing is in flux and in general the previous intense schedules are being revised downward.75 Many centers concentrate observations into the highest period of risk and use progressively less CT scans in follow-up. Many schedules in seminoma are recommending a 3–2–1 with 3 abdominal/pelvic CTs the first year, 2 the second, and 1 the third with no CTs thereafter. Thus, although surveillance is a very attractive option, the frequency of follow-up imaging and tests cannot be reduced in any given patient. Therefore, patients who elect surveillance need to be compliant and reliable.
Patients with stage I seminoma have been successfully treated with orchiectomy and adjuvant radiation for over 60 years with overall survival rates now above 99%. Adjuvant PA radiation results in a 3–4% rate of relapse with most of those being salvageable with additional radiation and/or chemotherapy. Analogous to the movement for surveillance is a simultaneous drive to reduce the toxicity of initial adjuvant therapy by reducing radiation dose and volume treated.
Radiation dose has been gradually reduced to 20–25.5 Gy in 1.5–2 Gy fractions in current practice. The MRC TE18/EORTC 30942 trial addressed whether the radiation dose could be safely lowered from 30 to 20 Gy.103 Most patients received only radiation to the PA nodes, and they were randomized to 30 Gy in 15 fractions versus 20 Gy in 10 fractions. The 0.7% absolute difference in relapse rates was statistically insignificant, and the lower dose was associated with less lethargy and inability to carry out normal work at 1 month.
Similarly, radiotherapy volume has been reduced.104 Ipsilateral pelvic lymph node irradiation did not significantly improve the 96% relapse-free survival at 3 years obtained with PA node field only radiation. However, four of nine of the patients who had been treated with PA node radiation had pelvic node involvement as a component of their relapse. Thus, patients who receive PA node radiation alone must still be followed with CT scans of the pelvis. Current practice is to treat the PA nodes from the top of T11 or T12 to the bottom of L5. Ipsilateral pelvic lymph node irradiation is still recommended, however, for patients who have had prior pelvic or inguinal surgery as this can disrupt normal lymphatic flow.PA node radiation alone is sufficient for patients with an undisturbed testicular lymphatic drainage.
Postorchiectomy chemotherapy
A large randomized trial was conducted by the EORTC/MRC in which patients with well-staged clinical stage I seminoma were randomly allocated to standard prophylactic radiation therapy (primarily PA radiation at 20–30 Gy) or treatment with single-course single-agent carboplatin (AUC 7).105, 106 This trial was designed to exclude an absolute increase in relapse at 2 years of 3%. In a 1 : 2 randomization scheme, 560 patients were assigned to single-agent carboplatin, and 885 were assigned to standard radiation therapy. With median 4-year follow-up, the 3-year relapse-free survival rate was 95.9% for radiation compared with 94.8% for carboplatin (p = 0.31), the majority of relapses on the carboplatin arm occurring in the abdomen and almost all of the recurrences in the radiation arm occurring outside the radiation portal. There were fewer new second primary TGCTs with carboplatin (two patients) versus radiotherapy (10 patients). The authors’ conclusions were that single-agent carboplatin was a safe and effective adjuvant treatment of clinical stage I seminoma, but follow-up longer than 4 years is necessary. Criticisms of the study were that the pattern of abdominal relapse with the carboplatin-treated patients may necessitate abdominal imaging in follow-up and that short follow-up may underestimate the true incidence of relapse. This trial was updated,106 and with median 6.5 year follow-up, there remains no significant difference in 5-year relapse-free survival between carboplatin (95%) and radiotherapy (96%). The rate of new second primary GCTs was lower with carboplatin (two patients) versus radiotherapy (15 patients).
Consensus guidelines in Europe and in Canada reflect the collected work of experienced radiation oncologists, medical oncologists, and urologists. The European consensus statement favors surveillance for all patients independent of the individual risk for relapse. Only if surveillance is not applicable, the equally effective alternatives are either adjuvant radiation or adjuvant carboplatin. A risk-adapted strategy with selection of adjuvant treatment according to individual risk is currently being investigated and is still experimental.107
Stage II seminoma
Several different staging systems have defined stage II seminoma, depending on how the distinctions are made for nodal size. Regardless of the specific staging system used, the potential for supradiaphragmatic involvement increases with the size of the subdiaphragmatic disease. At most centers, the arbitrary cutoff for systemic chemotherapy is 5 cm, while patients with nodal masses <5 cm can be treated with radiation alone. Unlike with stage I, surveillance is not an option, and the expected 5-year relapse-free survival drops to 89–95% for patients treated with radiation alone.108–110 The overall survival still remains around 97–99% because of the effectiveness of salvage therapy. For patients with stage II seminoma, the standard radiation field incorporates the PA and ipsilateral pelvic lymph nodes. The treatment of stage II seminoma with radiation has also evolved. Historically for a patient with stage II seminoma, radiation was delivered to the PA nodes, to the pelvic lymph nodes, and prophylactically to the mediastinum. This practice, although effective at limiting supradiaphragmatic failures, became associated with cardiac morbidity. Chemotherapy is now favored for patients who are at significant risk of supradiaphragmatic involvement.
There is also an increasing experience with chemotherapy as primary management of small- to moderate-volume stage II seminoma.111 A Spanish collaborative study entered 72 patients at 26 participating centers. Eighteen patients had stage IIA disease, and 54 patients had stage IIB disease. Eighty-three percent of patients achieved complete response (CR), and 17% achieved partial response (PR) with residual mass. After a median follow-up of 71.5 months, six patients with stage IIB disease experienced relapse, and one of these died of seminoma. Three patients experienced nonseminoma-related deaths. The estimated 5-year progression-free survival rates for patients with stage IIA or IIB disease were 100% and 87% (95% CI, 77.5–97%), respectively. Five-year progression-free and overall survival rates for the whole group were 90% (95% CI, 82–98%) and 95% (95% CI, 89–100%), respectively; mild to moderate emesis, stomatitis, and diarrhea were the most common nonhematologic effects. Such experiences strongly suggest that primary chemotherapy may be an effective alternative to abdominal radiation.
Therapy for disseminated disease
Chemotherapy: pre-cisplatin
A broad spectrum of chemotherapeutic agents were tested from 1952 to 1972 in disseminated GCTs. In the early 1960s, the vinca alkaloids were tested. Vinblastine induced complete remissions in 4 of 30 treated patients.109 Although these were of short duration, they provided investigators with a rational component of subsequent combination regimens.
Several antitumor antibiotics were also found to have single-agent activity, including actinomycin D. In 1970, bleomycin was reported to induce complete remissions.112, 113
An early combination regimen described in 1960, which contained methotrexate, actinomycin D, and chlorambucil, remained popular throughout the rest of the decade.114 Li reported an objective response rate of 52%, with more than half of those being CR. These were the first durable remissions, with five patients alive at 9–39 months after treatment.
A further advance occurred when vinblastine and bleomycin were used in combination.115 This regimen resulted in 65% of patients achieving a complete remission, including some durable responses.
The most important event in the development of curative therapy for disseminated disease was the 1965 report by Rosenberg and colleagues of the antibacterial effect of platinum coordination compounds.116 One of these compounds, cis-diamminedichloroplatinum (II) (cisplatin) was found also to have significant antitumor activity. Preclinical studies revealed testicular atrophy as a side effect in the dog model, prompting some investigators to predict activity for this compound against human testicular malignancies in the clinical setting. Cisplatin was soon reported to be the single most active agent against TGCTs, with response rates of 70% and complete remission rates of 50%.114 Thirty years later, it remains the most active drug in GCTs.
Indiana University studies
In 1974, investigators at Indiana University began studies using vinblastine (PVB) in patients with disseminated testicular cancer. The initial study remains a landmark study in modern oncology.117 This study incorporated principles of combination chemotherapy, as well as the concept of surgically resecting residual disease after the completion of chemotherapy. Induction chemotherapy was brief (three to four courses), and maintenance chemotherapy was given for 2 years after induction therapy. Thirty-three of 47 patients (70%) obtained a complete remission with chemotherapy. Of the remaining 14 patients, five obtained a complete remission with resection of residual disease. Six of the patients attaining remission relapsed.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree