INTRODUCTION
Testicular germ cell tumors (GCTs) account for the majority of testicular cancers and are highly curable. This chapter primarily discusses GCTs arising in the testicle, dividing this category into seminoma versus nonseminoma germ cell tumors (NSGCTs). Then, the rare entity of extragonadal GCTs, which can arise in the mediastinum, retroperitoneum, or pineal body, is described.
OVERVIEW OF GERM CELL TUMORS
The GCTs are the most common new cancer diagnosis in young men. Roughly an estimated 8,430 new cases were expected to be diagnosed in 2015 (1). Highlighting the high curability of this cancer, GCTs only claimed approximately 380 lives in 2015 (1) and carry a 5-year overall survival (OS) rate of approximately 95% (2,3). The GCTs have a bimodal age distribution, with most men diagnosed between ages 15 and 25. There is a second peak of diagnosis around age 60, which largely represents seminoma histology and a lower mortality risk. Lifetime risk for the development of GCTs is approximately 0.5% or 1 in 200 (4).
Worldwide, GCTs are six times more common in developed countries, with the largest incidence reported in Denmark and Switzerland and the lowest in Japan, Finland, and Israel (4). In the United States, the overall incidence of GCTs appears to be gradually increasing. The incidence has specifically increased among African Americans, with the greatest increase in seminoma histology. This does not appear to be related to screening or earlier diagnosis (5). Caucasian men, although still representing the group most likely to be diagnosed, are more likely to be identified at an earlier stage than in the past (6).
Cryptorchidism is one of the few identifiable risk factors for the development of GCTs, although representing at most about 10% of cases. When present, cryptorchidism imparts a relative risk between 2.5 and 17.1 (7,8). This increased risk includes the contralateral testicle, even if descended normally or via orchiopexy. It is unclear if orchiopexy reduces the lifetime risk of GCTs, although data showing increased incidence even in the contralateral testicle support the theory that the etiology of GCTs lies in abnormal gonadal development rather than anatomic malposition (9,10). Men with a prior history of GCTs also have an increased risk of GCTs in the contralateral testicle, suggesting a genetic predisposition, although men with a family history of GCTs account for only 1.5% of patients with new diagnosis (11). A personal history of GCT carries an increased lifetime risk of secondary cancers, irrespective of histologic type (12).
The most common genetic abnormality found in GCTs is an isochromosome of the short arm of chromosome 12, which has been identified in approximately 80% of GCTs (13). This abnormality can be found in all histologic subtypes except spermatocytic seminoma (14,15). Overexpression of c-kit is seen in seminoma (16). Of note, p53 is rarely altered in GCTs, and single-gene mutations in general are uncommon (17).
Carcinoma in situ (CIS), or intratubular germ cell neoplasia (ITGCN), has been identified as the precursor lesion in most GCTs. It is histologically described as atypical germ cells in the seminiferous tubules. Such changes are found adjacent to most invasive GCTs, with the notable exception of spermatocytic seminoma. The ITGCN cells express numerous proto-oncogenic proteins that play a role in tumorigenesis, including the receptor tyrosine kinase CD-117 or c-kit, a protein normally involved in germ cell migration and early differentiation (18,19).
The main histologies encountered in GCTs are seminoma, embryonal carcinoma, endodermal sinus tumor (EST, also known as yolk sac tumor), choriocarcinoma, and teratoma. The last can be further classified as mature, immature, or teratoma with malignant transformation. It is common to see more than one histologic subtype within a tumor. Importantly, the clinical course can be largely inferred from the histology. The GCTs that show exclusively the seminoma histology constitute pure seminomas, while those containing any other histologic pattern are classified as NSGCTs, even if the dominant histologic pattern is seminoma. Thus, the term seminoma is used in two very different ways: as a histologic pattern and as a main subdivision of GCTs. The biology and clinical expression are dominated by the nonseminoma component, and thus the presence of any histologic component other than seminoma places the tumor in the category of NSGCT.
Most patients with GCTs present with painless testicular swelling or a nodule. In some cases, testicular swelling can be accompanied by pain secondary to bleeding or infarction within the tumor. In the presence of pain or a history of injury, an appropriate differential diagnosis would include testicular torsion, epididymitis, orchitis, hydrocele, spermatocele, and hematoma. It is extremely important that regardless of pain or other associated symptoms, all scrotal masses should be approached as if they were malignant. In patients who present with gynecomastia, especially bilateral, GCTs should be considered (20). Other symptoms can include fever, weight loss, back pain, and hemoptysis (most often seen in patients presenting with high-volume disease).
The importance of prompt diagnosis and treatment cannot be stressed enough because the extent of disease at presentation predicts overall prognosis. Awareness of GCT prevalence among young men is important for both general practitioners and the general public. Radiographic evaluation of a suspected primary should include high-resolution, trans-scrotal ultrasonography with color Doppler of both testicles, and any suspicious lesion should be definitively evaluated with radical orchiectomy.
Trans-scrotal biopsy is contraindicated in the diagnostic workup of a suspected testicular neoplasm, as this procedure can disrupt regional lymphatics, potentially altering the otherwise-predictable nodal spread. Because the diagnosis of GCTs is rarely in question, the preferred diagnostic and therapeutic procedure for a testicular mass is radical inguinal orchiectomy. If a tissue diagnosis is felt to be necessary prior to orchiectomy, an open biopsy should be performed via an inguinal incision to allow for proper examination and tissue sampling with minimal risk of inguinal or scrotal contamination.
Serum markers, specifically human chorionic gonadotropin (hCG), α-fetoprotein (AFP) and lactate dehydrogenase (LDH), have unique diagnostic and prognostic significance in GCTs. These markers enable the clinician to infer clinical behavior, monitor therapy, decide when to apply surgical consolidation, and detect residual or recurrent disease.
Elevated in pregnancy, hCG is not normally detectable in males except in the setting of GCTs. With a half-life of 18 to 36 hours, hCG can also be markedly elevated in gestational trophoblastic disease and occasionally in epithelial cancers (21). It is composed of two subunits, α and β, which exist in multiple isoforms. The α subunit has sequence similarity to the α subunit of thyroid-stimulating hormone (TSH), follicle-stimulating hormone (FSH), and luteinizing hormone (LH), which leads to “cross-talk” between these hormones and hCG. For this reason, hCG assays measure the β subunit. This “cross talk” can be clinically significant in high-volume disease accompanied by high levels of hCG, where hCG causes hyperthyroidism by binding to the TSH receptor. Extreme elevation of hCG in males should be considered pathognomonic for GCTs and, in selected cases of threatening disease, justifies initiation of therapy even before tissue confirmation.
Normally produced by the fetal yolk sac, AFP also exists in multiple isoforms. It is elevated in GCT cells derived from the embryological yolk sac, including endodermal sinus tumor and embryonal carcinoma. It has also been found to be elevated in other neoplasms, such as hepatocellular carcinoma and pancreatic, gastric, and lung cancer, and has a serum half-life of approximately 5 days (22). Seminoma does not produce AFP, and in the setting of GCT, an elevated AFP implies a nonseminomatous histology (23).
Lactate dehydrogenase is neither cancer specific nor germ cell specific. Of the LDH isoforms, LDH-1 is most specific for GCTs; however, there is no established routine use for the fractionation of LDH and precise measurement of LDH-1. Total LDH can be used to estimate the prognosis in advanced NSGCT at the time of diagnosis or to detect recurrent disease (24).
The GCTs follow a distinct pattern of spread and metastasis. The lymphatic drainage from the testicle reflects embryologic origin, and thus the right testicle drains to the interaortocaval lymph nodes, and the left testicle drains to the left para-aortic lymph nodes. These initial nodes of spread are termed the “landing zone.” Epididymal lymphatics drain via the external iliac chain and scrotal lymphatics via the pelvic chain; therefore, locally advanced disease (involving the epididymis and scrotum) can present with involvement of these nodal basins. Distant metastasis involves the lungs principally, followed by the liver, brain, and bones.
STAGING
Table 39-1 shows the American Joint Commission on Cancer (AJCC) TNM staging of testicular cancer (25). This system is based on the anatomic characteristics of the tumor, the presence of elevated tumor markers, and the presence of distant disease. These well-defined risk factors are used to group patients into stages I-III. In general, stage I disease is confined to the testis, stage II disease has nodal metastases confined to the retroperitoneum with markers in the good prognosis range (S1), and stage III disease includes nodes that extend beyond the retroperitoneum, extranodal metastases, or elevation of tumor markers to the intermediate- or poor-prognosis range (S2-S3).
| Primary Tumor (T) | ||||
| The extent of primary tumor is usually classified after radical orchiectomy, and for this reason a pathologic stage is assigned. | ||||
| PTX | Primary tumor cannot be assessed | |||
| pT0 | No evidence of primary tumor (eg, histologic scar in testis) | |||
| PTis | Intratubular germ cell neoplasia (carcinoma in situ) | |||
| pT1 | Tumor limited to the testis and epididymis without vascular/lymphatic invasion; tumor may invade into the tunica albuginea but not the tunica vaginalis | |||
| pT2 | Tumor limited to the testis and epididymis with vascular/lymphatic invasion, or tumor extending through the tunica albuginea with involvement of the tunica vaginalis | |||
| pT3 | Tumor invades the spermatic cord with or without vascular/lymphatic invasion | |||
| pT4 | Tumor invades the scrotum with or without vascular/lymphatic invasion | |||
| Regional Lymph Nodes (N) Clinical | ||||
| NX | Regional lymph nodes cannot be assessed | |||
| N0 | No regional lymph node metastasis | |||
| N1 | Metastasis with a lymph node mass ≤2 cm in greatest dimension or multiple lymph nodes, none >2 cm in greatest dimension | |||
| N2 | Metastasis with a lymph node mass >2 cm but not >5 cm in greatest dimension; or multiple lymph nodes, any one mass >2 cm but not >5 cm in greatest dimension | |||
| N3 | Metastasis with a lymph node mass >5 cm in greatest dimension | |||
| Pathologic (PN) | ||||
| PNX | Regional lymph nodes cannot be assessed | |||
| pN0 | No regional lymph node metastasis | |||
| pN1 | Metastasis with a lymph node mass ≤2 cm in greatest dimension and ≤5 nodes positive, none >2 cm in greatest dimension | |||
| pN2 | Metastasis with a lymph node mass >2 cm but not >5 cm in greatest dimension; or >5 nodes positive, none >5 cm; or evidence of extranodal extension of tumor | |||
| pN3 | Metastasis with a lymph node mass >5 cm in greatest dimension | |||
| Distant Metastasis (M) | ||||
| M0 | No distant metastasis | |||
| M1 | Distant metastasis | |||
| M1a | Nonregional nodal or pulmonary metastasis | |||
| M1b | Distant metastasis other than to nonregional lymph nodes and lungs | |||
| Serum Tumor Markers (S) | ||||
| SX | Marker studies not available or not performed | |||
| S0 | Marker study levels within normal limits | |||
| S1 | LDH <1.5 × N AND | |||
| hCG (mIU/mL) <5,000 AND | ||||
| AFP (ng/mL) <1,000 | ||||
| S2 | LDH >1.5-10 × N OR | |||
| hCG (mIU/mL) 5,000-50,000 OR | ||||
| AFP (ng/mL) 1,000-10,000 | ||||
| S3 | LDH >10 × N OR | |||
| hCG (mIU/mL) >50,000 OR | ||||
| AFP (ng/mL >10,000 | ||||
| N indicates the upper limit of normal for the LDH assay. | ||||
| Stage Grouping | ||||
| Stage 0 | pTis | N0 | M0 | S0 |
| Stage I | pT1-pT4 | N0 | M0 | SX |
| Stage IA | pT1 | N0 | M0 | S0 |
| Stage IB | pT2 | N0 | M0 | S0 |
| pT3 | N0 | M0 | S0 | |
| pT4 | N0 | M0 | S0 | |
| Stage IS | Any pT/Tx | N0 | M0 | S1-S3 |
| Stage II | Any pT/Tx | N1-N3 | M0 | SX |
| Stage IIA | Any pT/Tx | N1 | M0 | S0 |
| Any pT/Tx | N1 | M0 | S1 | |
| Stage IIB | Any pT/Tx | N2 | M0 | S0 |
| Any pT/Tx | N2 | M0 | S1 | |
| Stage IIC | Any pT/Tx | N3 | M0 | S0 |
| Any pT/Tx | N3 | M0 | S1 | |
| Stage III | Any pT/Tx | Any N | M1 | SX |
| Stage IIIA | Any pT/Tx | Any N | M1a | S0 |
| Any pT/Tx | Any N | M1a | S1 | |
| Stage IIIB | Any pT/Tx | N1-N3 | M0 | S2 |
| Any pT/Tx | Any N | M1a | S2 | |
| Stage IIIC | Any pT/Tx | N1-N3 | M0 | S3 |
| Any pT/Tx | Any N | M1a | S3 | |
| Any pT/Tx | Any N | M1b | Any S | |
Of particular importance to men with GCTs is the preservation of fertility. Both the diagnosis and treatment of GCTs are associated with impaired fertility. It is recommended that, if clinically feasible, the patient be counseled about and offered the opportunity to pursue sperm banking prior to starting chemotherapy. It is not recommended to delay chemotherapy in symptomatic poor-risk patients, as poor physical condition often makes sperm donation difficult or even impossible (26).
TESTICULAR SEMINOMA
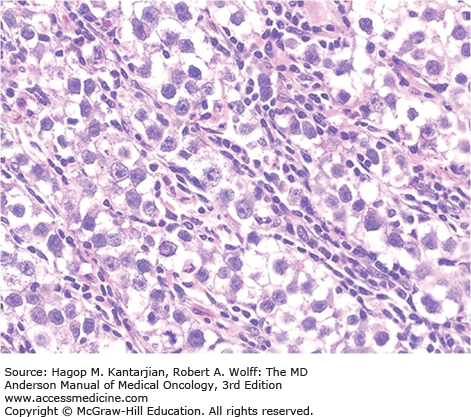
Under microscopic visualization, classic seminoma has a “fried-egg” appearance, defined as a monotonous proliferation of large, rounded cells arranged in sheets or cords with large centralized nuclei and nucleoli. These tumors can be difficult to distinguish from lymphoma if there is a background of lymphocytic infiltration. Further confirmation (ie, negativity for lymphocyte markers such as common leukocyte antigen) is often required. Although not specific, seminomas stain positive for placental alkaline phosphatase (PLAP) and are routinely negative for AFP and hCG. Figure 39-1 shows the histological appearance of classic seminoma.
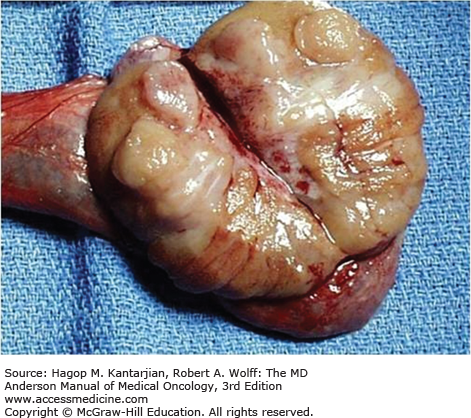
On pathologic examination of the testis, seminoma tends to be a semisolid tumor that readily oozes onto the gross examination table. This makes the presence of malignant cells on the surface of the spermatic cord and at the margins of resection a ubiquitous finding. Thus, the clinician must be careful not to be unduly influenced by reports of “margin positivity” and “involvement of the spermatic cord” in the pathology report (27). Figure 39-2 shows the typical gross appearance of seminoma.
Even in the presence of significant metastatic disease, it is not uncommon to find only a scar in the testicle. This phenomenon is known as “burned-out” seminoma and can also occur in NSGCT. The biological basis for this spontaneous regression of the primary is not known. Seminomas are typically associated with significant inflammatory infiltration, and metastatic deposits characteristically leave a dense desmoplastic residual mass after treatment, often making them difficult to resect.
Pure seminoma is the most common GCT of the testicle, accounting for approximately 50% of GCTs. By definition, seminomas have no evidence of a nonseminoma component and do not produce AFP but may have modest elevation of β-hCG. Spermatocytic seminomas, a rare variant comprising only 10% of seminomas, are not associated with ITGCN. These tumors typically occur in men over 50 years old, in stage I disease, and have a low metastatic rate. This subtype portends an excellent prognosis with resection alone (with or without radiotherapy) (28). Seminomas tend to spread via lymphatics initially, with late hematogenous spread, and are more likely to spread locally, as evidenced by positive margins and involvement of the spermatic cord on histology. The most common hematogenous spread is to the lungs, and metastatic seminomas rarely metastasize to the brain. Remarkably, bulky tumors rapidly respond, with dramatic loss of tumor bulk, but tumor lysis syndrome is never encountered.
The International Germ Cell Cancer Collaborative Group (IGCCCG) established a standard risk classification for both seminomas and NSGCTs (Table 39-2). Patients with metastatic seminoma are divided into either good- or intermediate-risk categories, with no definable “poor-risk” seminoma. The one characteristic that predicted worse outcome for seminoma was the presence of nonpulmonary visceral metastases (intermediate prognosis). The prechemotherapy tumor markers do not predict prognosis (unlike in NSGCTs, discussed further in the chapter). Ninety percent of patients with seminoma fall into the good-prognosis category, with a 5-year OS of 86%. Patients with seminoma in the intermediate-prognosis category have a 5-year OS of 72% (2).
| Seminoma | Nonseminoma |
|---|---|
| Good Risk | |
| Any primary site | Testis/retroperitoneal primary |
| and | and |
| No nonpulmonary visceral metastases | No nonpulmonary visceral metastases |
| and | and |
| Normal AFP, any hCG, any LDH | AFP <1000 ng/mL |
| hCG <5000 mIU/mL | |
| LDH <1.5 × ULN | |
| 82% 5-year PFS; 86% 5-year OS | 86% 5-year PFS; 90% 5-year OS |
| Intermediate Risk | |
| Any primary site | Testis/retroperitoneal primary |
| and | and |
| Nonpulmonary visceral metastases | No nonpulmonary visceral metastases |
| and | and |
| Normal AFP, any hCG, any LDH | AFP 1,000-10,000 ng/mL |
| hCG 5,000-50,000 mIU/mL | |
| LDH 1.5-10 × ULN | |
| 67% 5-year PFS; 72% 5-year OS | 75% 5-year PFS; 80% 5-year OS |
| Poor Risk | |
| — | Mediastinal primary |
| or | |
| — | Nonpulmonary visceral metastases |
| or | |
| AFP >10,000 ng/mL | |
| — | hCG >50,000 mIU/mL |
| LDH >10 × ULN | |
| — | 41% 5-year PFS; 48% 5-year OS |
Patients with clinical stage I seminoma, representing 70% of patients at diagnosis, have disease confined to the testicle with no evidence of nodal or distant metastasis. Most patients will be cured by radical orchiectomy alone, but approximately 20% recur without adjuvant intervention. The disease-specific survival is nearly 100% with or without adjuvant treatment because patients who recur on surveillance are readily salvaged with standard treatment.
The benefits of surveillance include avoidance of unnecessary treatment in patients who are likely to be already cured by orchiectomy. Warde et al reported data on 638 patients with clinical stage I seminoma managed with surveillance with a median follow-up of 7 years. Patients with a primary tumor less than 4 cm maximum dimension and without invasion of rete testis had 5-year risk of relapse of only 12%. Patients with both risk factors had a risk of recurrence of 32%, while one of the two risk factors portends a 16% risk of relapse (29). Because of excellent outcomes of patients later treated for recurrent disease, active surveillance is considered a reasonable option for most patients, including those with both risk factors.
The recurrence rate after prophylactic radiotherapy for clinical stage I seminoma is about 4%, and most of those patients who recur after radiation survive with additional treatment (chemotherapy). Treatment of para-aortic lymph nodes to a dose of 20 Gy was associated with excellent local control approaching 100%. A randomized trial of 20 Gy versus 30 Gy showed no difference in rate of recurrence. Omission of ipsilateral iliac lymph nodes from the treatment field resulted in less toxicity (infertility, gastrointestinal effects) and minimal loss of efficacy. Radiotherapy is contraindicated for patients with horseshoe kidney or inflammatory bowel disease.
Radiotherapy was once viewed favorably because it reduced the number of computed tomographic (CT) scans that were necessary for follow-up, with a net reduction in the cost of treatment. There is, however, a risk of second malignant neoplasms related to treatment. Studies of testicular cancer survivors 25 or more years after treatment have revealed an increase in midline cancers, such as gastrointestinal and genitourinary malignancies. This added risk has brought about a reassessment of whether radiotherapy is warranted, especially considering that 80% of patients with clinical stage I seminoma will be treated unnecessarily, and that there is no survival benefit. At MD Anderson Cancer Center (MDACC), we no longer offer prophylactic radiation to men with stage I testicular seminoma.
A randomized controlled trial was conducted to compare a single infusion of carboplatin, with dose based on area under the curve (AUC) of 7, versus radiotherapy for the adjuvant treatment of clinical stage I seminoma (30). Median follow-up was 4 years, and the relapse-free survival was similar in both treatment arms, 96.7% and 97.7%, respectively, showing noninferiority of the one-cycle, single-agent carboplatin. There are limited data comparing the long-term safety of carboplatin to that of radiotherapy, leading many practitioners to adopt surveillance as the preferred option. Figure 39-3 outlines an approach to therapy for stage I seminoma.
FIGURE 39-3
Management of testicular cancer (seminoma). BEP, bleomycin, etoposide, and cisplatin; EP, etoposide and cisplatin; TIP, paclitaxel, ifosfamide, cisplatin; VIP, etoposide, ifosfamide, cisplatin.