Key points
- •
Cytotoxic chemotherapy has not proven effective in hepatocellular carcinoma (HCC). Sorafenib is the only approved agent for advanced, unresectable disease.
- •
Current studies are evaluating a wide range of targeted agents covering the spectrum of molecular aberrations in HCC. Currently, there are no effective alternatives for sorafenib-refractory HCC.
- •
The combination of sorafenib and chemoembolization has not been shown to improve clinical outcomes. Combined systemic and liver-directed therapy in HCC remains investigational.
Introduction
Research and therapeutic development in advanced hepatocellular carcinoma (HCC) have evolved in recent years. New insights into the genetics and molecular biology of HCC have transformed the way it is studied and treated.
The management of HCC is generally determined by disease extent and performance status with the additional consideration of hepatic function. The prototypical Barcelona Clinic Liver Cancer (BCLC) algorithm considers these variables in separating patients into discrete groups for which specific lines of therapy are prescribed. However, these categories are more fluid in daily clinical practice, especially for locally advanced disease considered unresectable. These patients may be treated with locoregional and/or systemic therapies at different times during the clinical course rather than pursuing a strictly linear therapeutic sequence. The growing reality of an interdisciplinary management approach is reflected by the proliferation of clinical trials studying combined modality therapy.
This chapter will discuss the management of locally advanced HCC that is no longer amenable to surgical and liver directed ablative therapies, as well as metastatic disease. An overview of systemic therapies currently being investigated in HCC will be presented in addition to a discussion of aspects that are important for the interventional radiology domain.
Staging
The challenge of managing HCC arises from the dual nature of the disease: the malignancy itself and the underlying cirrhosis. Before embarking on any course of treatment, an assessment of the hepatic functional reserve is imperative, in view if its prognostic implications, and its impact on the management plan. The most commonly used scoring system for cirrhosis in clinical practice is the Child-Pugh classification, which uses three laboratory (serum bilirubin, albumin, international normalized ratio [INR]) and two clinical (ascites, encephalopathy) variables. Each variable is graded according to its severity. The total grades of all five variables are added and make up a score that ranges from the least decompensated category (A), through moderate (B), to the most decompensated (C) liver.
The key limitation of the Child-Pugh scoring system is its lack of any cancer-related parameters, which obviously are key contributors to HCC patients’ prognosis.
The objective of a staging system is to provide prognostic information that helps the clinician devise a management plan appropriate for the anticipated clinical outcome. Several different staging systems have been developed for HCC that include the purely anatomic American Joint Committee on Cancer/Union Internationale Contre le Cancer (AJCC/UICC) TNM (tumor, node, metastasis) system to a variety of hybrid anatomic and functional systems such as the BCLC algorithm. Given that each of these systems were developed in populations of varying ethnicities, risk factors for cirrhosis, and treatment approaches, their general applicability and predictive potential, especially for patients with locally advanced or unresectable HCC, is a continued subject of debate. Although the BCLC algorithm is considered to be the most comprehensive of the hybrid staging systems and has been validated prospectively, , its discriminatory power among patients with advanced HCC is limited. A single-institution review of the different systems found that the Cancer of the Liver Italian Program (CLIP) which was developed in patients with hepatitis C, the Chinese University Prognostic Index (CUPI) developed primarily in patients with hepatitis B, and the Groupe d’Etude et de Traitement du Carcinome Hepatocellulaire (GETCH) best predicted outcomes in patients with advanced HCC, whereas the BCLC and sixth edition TNM systems were not informative in this setting. These findings were consistent with an earlier study showing that the CLIP system outperformed the BCLC and Okuda systems in the palliative setting. Thus, although the BCLC algorithm serves as an important roadmap for prognostication and management, outcomes of patients with locally advanced or metastatic HCC may be better estimated by the CLIP, CUPI, or GETCH systems if the dominant underlying risk factor is hepatitis C, hepatitis B, or alcoholic cirrhosis, respectively.
Systemic chemotherapy
Several classes of chemotherapy agents exist, each exerting its cytotoxic effects through one or several of these mechanisms: alkylating agents, platinums, and topoisomerase inhibitors cause DNA strand breakage; fluoropyrimidines and other antimetabolites inhibit DNA synthesis; taxanes and vinca alkaloids prevent microtubule formation and cell division. Unlike molecularly targeted agents that will be discussed later, chemotherapy represents a less discriminatory form of therapy; normal cells rely on the same mechanisms to maintain DNA integrity, proliferate, and survive as their cancerous counterparts and are therefore subject to the same cytotoxic effects. Many of the toxicities of chemotherapy drugs result from the collateral damage incurred to normal cells in addition to cancer cells.
Studies of single-agent chemotherapy in HCC began during the 1960s–1970s. One of the earliest agents to be tested was doxorubicin (also known as adriamycin), from the anthracycline family of alkylating agents. In 1975, a phase 2 trial reported a striking objective response rate of 79% and median survival of 8 months. Subsequent studies of doxorubicin were unable to replicate these results; response rates ranged from 15% to 20% and median survival was only 4 months. Phase 3 studies also did not show doxorubicin to be superior to other chemotherapy regimens.
Clinical trials of 5-fluorouracil, a fluoropyrimidine used in the treatment of many gastrointestinal malignancies, as well as newer agents such as the topoisomerase I inhibitor irinotecan and the antimetabolite gemcitabine failed to produce durable response and survival outcomes. Although combination regimens such as gemcitabine + oxaliplatin (GEMOX) and oral 5-fluorouracil (capecitabine) + oxaliplatin (CAPOX) appeared to be more active on the basis of phase 2 data, their impact on survival has not been assessed in phase 3 studies. The strategy of combined chemoimmunotherapy has also been studied in advanced HCC. Interferon α-2b (IFN) was combined with cisplatin, doxorubicin, and 5-fluorouracil (PIAF) in a phase 2 study. Median survival was 8.9 months. Notably, 26% of patients had a partial response, 12% were converted to resectable disease, and 8% of patients had a pathologic complete response. PIAF was associated with significant hematologic toxicities; 34% of patients had grade 3/4 leukopenia, 22% had grade 3/4 thrombocytopenia and two patients died of neutropenic sepsis. Despite the considerable toxicities, the compelling clinical and pathologic responses reported in this study led to a phase 3 trial comparing PIAF with doxorubicin, which was still considered the most effective chemotherapy agent at the time. The primary endpoint, overall survival, was not statistically significantly different between the study arms (8.7 vs. 6.8 months, P = .83). Response rates were higher but not statistically significant in favor of PIAF (20.9% vs. 10.5%, P = .06), and one patient on each arm had a pathologic complete response. There was a significantly higher incidence of neutropenia, thrombocytopenia, and hypokalemia of any grade on the PIAF arm; however, there were no treatment-related deaths.
The limited impact of chemotherapy on responses and survival in HCC has several potential explanations. HCC tumors have been shown to overexpress the multidrug resistance gene-1 ( MDR-1 ) and P-glycoprotein drug extrusion molecule. Furthermore, the therapeutic index of chemotherapy, especially chemoimmunotherapy, is narrowed in a patient population with limited end-organ reserve. For these reasons, chemotherapy has been supplanted by molecularly targeted agents in HCC, though it may still be useful as an adjunct to these agents. The combination of sorafenib and doxorubicin has shown promising activity and will be discussed below.
Targeted therapies
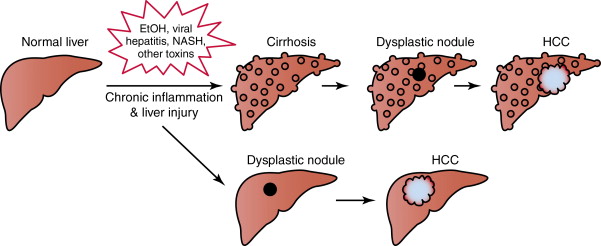
In most cases, the initiating step in hepatocarcinogenesis is liver injury due to hepatotoxins, viral hepatitis, or other insults, leading to chronic inflammation, cirrhosis, and the formation of dysplastic nodules and ultimately HCC ( Figure 9-1 ). These steps are accompanied and driven by amplifications in oncogenes, losses in tumor suppressor function, and changes in signaling pathways that are normally responsible for maintaining hepatohomeostasis. Among the myriad factors that have been implicated in this process are the receptor tyrosine kinases (RTKs) including the epidermal growth factor receptor (EGFR) family, insulinlike growth factor receptor (IGFR), vascular endothelial growth factor receptor (VEGFR), and c-met. Interactions between these cell surface molecules and their specific ligands cause a conformational change in the RTK, leading to phosphorylation of their intracellular domains, and downstream activation of signaling cascades including the Ras/Raf/Mek/Erk (MAP kinase) and PI3K/Akt/mTOR cascades. Signal transmission through these intracellular conduits results in the transcriptional activation of genes that govern cell growth, proliferation, the evasion of apoptosis, angiogenesis, and invasive and metastatic capabilities. Established HCC tumors also continue to evolve and acquire additional aberrations that enable their survival through compensatory changes in the same pathways, or through the co-optation of associated networks. Therapeutic disruption of tumor cell signaling circuitry at its different levels is actively being studied in HCC.
Receptor tyrosine kinases
Epidermal growth factor receptor
The EGFR superfamily consists of four RTKs: EGFR, c-erbB-2, c-erbB-3, and c-erbB-4, which regulate key cellular functions including proliferation, survival, angiogenesis, and motility. Recognition of the primacy of EGFR as an oncogene in multiple malignancies has made it a leading therapeutic target. Aberrant EGFR activity in HCC appears to be primarily the result of overexpression, which also appears to correlate with more aggressive disease characteristics. , Anti-EGFR agents undergoing active clinical trial assessment in HCC include small-molecule tyrosine kinase inhibitors (TKIs) and anti-EGFR monoclonal antibodies.
Small-molecule TKIs target the intracellular signaling domain of the EGFR, preventing the activation of downstream cascades and the transcription of genes that promote malignant behavior. Three anti-EGFR TKIs have been assessed in HCC: erlotinib, gefitinib, and lapatinib.
Erlotinib, a selective inhibitor of EGFR/ErbB-1/Her-1, was evaluated in two phase 2 studies that enrolled fit patients with locally advanced/metastatic HCC, 70%–80% of whom had Child-Pugh A liver function. The first study enrolled 38 patients, 50% of whom previously received systemic therapy. Progression-free survival, defined as the proportion of patients without disease progression by Response Evaluation Criteria in Solid Tumors (RECIST) guidelines or death, at 6 months was 32%. Nine percent of patients had a partial response lasting 2–11 months, 50% had stable disease, and median survival was 13 months. Similar findings were noted in a phase 2 study that enrolled 40 patients who were naïve to systemic therapy. Although no objective responses were observed, 43% of patients had stable disease; progression-free survival at 4 months was 42.5% and median survival was 10.8 months. The most common grade 3/4 toxicities with erlotinib were fatigue, diarrhea, skin rash, nausea, vomiting, anorexia, and transaminitis, which occurred in up to 13% of patients. In both studies, neither the presence nor the intensity of tumor EGFR immunoreactivity correlated with responses, and the relationship between cutaneous toxicity and clinical outcomes was not explored. ,
Gefitinib did not exhibit clinically relevant activity in a phase 2 study conducted in 31 patients with advanced disease naïve to systemic therapy. Although treatment was well tolerated, there was only one partial response, and 7 patients had stable disease for a disease control rate of 26%. Median progression-free and overall survival were 2.8 and 6.5 months, respectively
The dual EGFR/c-erbB-1 and c-erbB-2/Her-2 inhibitor, lapatinib, was evaluated in a phase 2 trial conducted in a mixed population of patients with advanced HCC and biliary tract carcinomas. The practice of evaluating therapies in studies that include both HCC and biliary tract carcinomas is fading away as we learn more about the different biology of those two diseases. Regardless, the Ramanathan study did not show any activity in the HCC subgroup; 5% of patients had an objective response, and median progression-free and overall survival were 2.3 and 6.2 months, respectively. Results were somewhat better in another phase 2 trial that enrolled 26 patients with advanced HCC, 19% of whom were previously treated. Although there were no objective responses and median progression-free survival was only 1.8 months, 40% of patients had stable disease (lasting >3 months in six patients and >1 year in two patients) and median overall survival was 12.6 months. Factors associated with improved survival included the development of a skin rash, infection with hepatitis C, and the presence of ≥20 EGFR intron 1 (CA) n16-23 repeats within tumor cells instead of <20 repeats.
Cetuximab is a chimeric monoclonal antibody that binds to the extracellular domain of the EGFR, preventing receptor-ligand interactions as well as intracellular domain phosphorylation. In a phase 2 study of cetuximab in HCC, there were no objective responses although 17% of patients had stable disease lasting a median of 4 months. Median progression-free and overall survival on that study were 1.4 and 9.6 months, respectively. Tumor EGFR expression did not correlate with responses to cetuximab. In another phase 2 study, 44.4% of patients had stable disease with a median time to progression of 8 weeks. Among patients who maintained stable disease for >8 weeks, the median time to progression was 22.5 weeks. Sixty percent of responders exhibited an upregulation of cell cycle inhibitory proteins compared to only 14% of nonresponders. The relationship between the development of skin rash, KRAS and BRAF mutation status, and responses to cetuximab has not been explored in HCC.
Taken together, anti-EGFR agents failed to show any meaningful activity in advanced HCC, although there appear to be subgroups of patients who might derive greater benefit from these agents compared to the unselected population. Characterization of these subgroups remains a work in progress.
Insulinlike growth factor
Obesity, dyslipidemia, type 2 diabetes, and nonalcoholic steatohepatitis (NASH) are features of the metabolic syndrome that is a recognized risk factor for the development HCC. The link between the metabolic syndrome and HCC is thought to be mediated by insulin, insulinlike growth factor (IGF) ligands and their receptors, in concert with reactive oxygen species, proinflammatory cytokines, and activation of the c-Jun amino-terminal kinase 1 (JNK1). These complex interactions result in a final common pathway of hepatocellular inflammation, apoptosis, compensatory proliferation and, ultimately, hepatocarcinogenesis. Targeting of the IGF axis using small-molecule TKIs and monoclonal antibodies is currently being evaluated in HCC.
A phase 2 study of the anti IGF-1 receptor (IGF-1R) antibody, cixutumumab (IMC-A12), in advanced HCC was terminated for inefficacy after 24 patients were enrolled. The primary endpoint, progression-free survival at 4 months, was 30% and median overall survival was 8 months. There were no objective responses, 29% of patients had stable disease, and 41% progressed. Therapy was complicated by grade 3 and 4 hyperglycemia in 46% of patients. High serum IGF binding protein-1 levels correlated positively with progression-free survival. The combination of cixutumumab and sorafenib in patients with advanced HCC naïve to systemic therapy is undergoing evaluation in both a phase 1 and phase 2 study ( www.clinicaltrials.gov , NCT00906373, NCT01008566).
BIIB022 and AVE 1642 are two other anti-IGF-1R antibodies undergoing evaluation in HCC. A phase 1B study of sorafenib and BIIB022 has been completed and results are pending ( www.clinicaltrials.gov , NCT00956436). The combination of AVE1642 and sorafenib was found to be safe and tolerable in a phase 1 study that also reported stable disease lasting a median of 13 weeks in 85% of patients.
OSI-906 is an oral dual inhibitor of IGF-1R and the insulin receptor. A randomized phase 2 study in advanced HCC was terminated by the sponsor ( www.clinicaltrials.gov , NCT01101906), but another phase 2 study assessing the combination of OSI-906 and sorafenib is ongoing ( www.clinicaltrials.gov , NCT01334710).
C-met and the hepatocyte growth factor
The c-met receptor tyrosine kinase and its cognate ligand, hepatocyte growth factor (HGF), are potent mitogens that coordinate hepatogenesis in the developing embryo and promote regeneration and maintain the integrity of the mature liver. In preclinical models, aberrant c-met expression demonstrates tumorigenic potential, and inactivation of c-met/HGF signaling has shown antitumor activity. , Although data on the clinicopathologic characteristics and outcomes associated with c-met overexpression in human HCC are conflicting, , c-met targeting is actively being evaluated in clinical trials. Tivantinib (ARQ197), an oral non-ATP-competitive c-met TKI, is currently being studied in a phase 1B study of patients with Child-Pugh A or B cirrhosis and HCC, with preliminary data demonstrating acceptable toxicity ( www.clinicaltrials.gov , NCT00802555). A phase 1 dose escalation study of ARQ197 and sorafenib in HCC has shown that the combination is well tolerated and may be active in treatment-naïve as well as pretreated disease ( www.clinicaltrials.gov , NCT00827177).
Several hybrid antiangiogenic and c-met inhibitors have also been developed and are being studied in HCC. Results from the HCC subset of a phase 2 discontinuation study of cabozantinib (XL184), a dual c-met/VEGFR-2 inhibitor were recently presented. Seventy-eight percent of patients experienced tumor regression with cabozantinib and median progression-free and overall survival were 4.4 months and 15.1 months, respectively. Tumor regression was associated with a ≥50% decrease in AFP from baseline in 35% of patients, and outcomes were not affected by prior therapy with sorafenib. Of note that tumor c-met expression was not assessed. Foretinib, another c-met/VEGFR-2 inhibitor, is also being studied in a phase 1/2 trial ( www.clinicaltrials.gov , NCT00920192).
Intracellular signaling cascades
Receptor tyrosine kinases such as EGFR, IGF-1R, c-Met, and, as will be later discussed, VEGF exert their biological effects by activating a complex network of intracellular signaling pathways. Two of the most extensively studied pathways in oncology are the Ras/Raf/Mek/Erk or MAPK/ERK cascade and the PI3K/Akt/mTOR cascade, both of which have been implicated in the pathogenesis of many malignancies.
In HCC, both the MAPK/ERK and PI3K/Akt/mTOR pathways are constitutively active. Aberrant MAPK/ERK activity may be induced by the effects of hepatitis B or C viral proteins on Raf kinase among other mechanisms. In addition, ERK 1/2 activation has also been associated with more aggressive disease behavior and poorer survival. Likewise, upregulation of various markers of mTOR activity has been associated with less favorable histopathologic features, more advanced disease, and both a shorter overall survival and time to recurrence after resection for HCC. , Activation of the PI3K/Akt/mTOR pathway has been documented in 40%–50% of HCC tumors. Mutations, downregulation, and deletions of the PTEN tumor suppressor gene are among the hypothesized mechanisms of PI3K/Akt/mTOR dysregulation in HCC.
Targeting the mapk/erk pathway
Functional Ras molecules are generated through posttranslational farnesylation of their C-termini. In preclinical studies, farnesyltransferase inhibitors exhibit chemopreventive properties and have been shown to reduce the proliferative and metastatic potential of HCC cells through inhibition of Ras as well as PI3K/Akt activation. A phase 1B trial of the farnesyltransferase inhibitor lonafarnib (SCH66336) with or without gemcitabine in advanced primary liver cancers was recently terminated for unspecified reasons ( www.clinicaltrials.gov , NCT00020774).
Farnesyl can also be manufactured through the mevalonate biosynthesis pathway, of which HMG CoA reductase is a component. Statins, which inhibit HMG CoA reductase, have been associated with a decreased incidence of HCC, particularly in diabetic patients and those infected with hepatitis B. , Randomized phase 2 and 3 clinical trials of sorafenib with or without pravastatin in advanced HCC are currently recruiting ( www.clinicaltrials.gov , NCT01075555, NCT01418729).
Selumetinib (AZD6244), a selective Mek1/2 inhibitor, was studied in a phase 2 trial that was terminated for inefficacy. No objective responses were seen, though 35% of patients had stable disease. Both the median time to progression and PFS were 1.4 months, and median survival was 4.2 months. Toxicities were mild; grade 3 and 4 elevations in liver enzymes, hyperbilirubinemia, fatigue, hyperglycemia, hypokalemia, and hyponatremia each occurred in 5% of patients.
A phase 1/2 clinical trial of selumetinib and sorafenib is currently ongoing ( www.clinicaltrials.gov NCT01029418). Another MEK inhibitor, BAY 86-9766, is also being combined with sorafenib in a phase 2 trial ( www.clinicaltrials.gov , NCT01204177). Another MEK inhibitor, BAY 86-9766, is also being combined with sorafenib in a phase 2 trial ( www.clinicaltrials.gov , NCT01204177).
Targeting the pi3k/akt/mTOR pathway
Clinical experience with mTOR inhibition in advanced HCC suggests disease-stabilizing activity in both the first-line setting and after progression on sorafenib. Phase 1/2 and 3 trials examining RAD001 monotherapy in the first- and second-line settings have been opened ( www.clinicaltrials.gov , NCT00390195, NCT00516165, NCT01035229), along with combination regimens of RAD001 with bevacizumab, sorafenib, or pasireotide ( www.clinicaltrials.gov , NCT00467194, NCT01005199, NCT01488487). Temsirolimus is also being assessed in combination with sorafenib in phase 1 and 2 studies ( www.clinicaltrials.gov , NCT01335074, NCT01013519) and with bevacizumab in a phase 2 trial ( www.clinicaltrials.gov , NCT01010126)
Antiangiogenesis
Angiogenesis is a complex, multifaceted process orchestrated by a myriad of growth factors and stimuli; vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), basic fibroblast-derived growth factor (bFGF), angiopoietins, and their cognate receptors as well as the oxygenation status of the tumor milieu are recognized contributors. Several of these factors have been found to be overexpressed and/or dysregulated in preneoplastic liver and established HCC. Systemic antiangiogenic therapies are a rapidly evolving area of research in HCC.
The different types of antiangiogenics.
VEGF signaling is the most extensively studied of the angiogenic pathways. Binding of VEGF to its cognate receptor, VEGF-R, induces intracellular signaling cascade activation and the transcription of genes controlling angiogenesis, mitogenesis, and survival. In HCC, baseline elevations in serum VEGF have been shown to correlate with unfavorable clinical and disease characteristics, and may also have independent prognostic value. Anti-VEGF agents are believed to exert their effects through several mechanisms: the disruption of blood vessel formation leading to tumor cell hypoxia and necrosis, and/or normalization of the vasculature which allows for more effective drug delivery to the tumors they feed.
Anti-VEGF(R) agents
Bevacizumab is a humanized anti-VEGF A monoclonal antibody that is U.S. Food and Drug Administration (FDA) approved for the treatment of advanced colorectal cancer, renal cell carcinoma, nonsquamous non-small-cell lung cancer and glioblastoma. In a phase 2 trial conducted in patients with localized HCC, objective responses were observed in 13% of patients, including one complete response. Median progression-free survival was 6.9 months and survival at 1 and 3 years was 58% and 23%, respectively. Hemorrhagic toxicity was a concern; 11% of patients experienced higher than grade 3 events and there was one fatal episode of variceal bleeding, prompting an amendment requiring that patients with a history of varices or evidence of varices on imaging undergo pretreatment endoscopies.
Ramucirumab is a recombinant human mAb that targets the VEGFR-2 receptor, preventing binding with VEGF. In a phase 2 first-line trial, ramucirumab produced a disease control rate of 50%, including partial responses in 3% of patients, and median progression-free survival was 4.3 months. Median progression-free survival was shorter in patients with Child-Pugh B liver function (2.8 months) than in those with Child-Pugh A liver function (4.2 months). Grade 3 adverse reactions included hypertension in 12%, gastrointestinal bleeding in 7%, infusion-related reactions in 5%, and headaches in 2% of patients.
Cediranib (AZD2171) is an oral small molecule inhibitor of VEGFR. Two phase 2 trials of cediranib in advanced, unresectable HCC were opened, one of which was suspended for unspecified reasons ( www.clinicaltrials.gov , NCT00427973). In the second trial, 84% of patients had grade 3 toxicities consisting primarily of hypertension, fatigue, and anorexia, resulting in a high attrition rate of 29%. Efficacy data are pending.
Targeting basic fibroblast growth factor
Upregulation of basic fibroblast growth factor (bFGF) can initiate tumorigenesis and is a purported mechanism of acquired resistance to VEGF inhibition. Brivanib is an orally administered dual inhibitor of VEGF and FGF signaling. In a phase 2 first-line study conducted in 55 patients with advanced HCC, the objective response rate was 7.2%, including one complete response, and 40% of patients had stable disease. Median and 6-month progression-free survival were modest at 18.2% and 2.7 months, respectively, and median overall survival was 10 months. Patients who experienced disease control had a longer survival time (15.2 months) than those who progressed (5.1 months, hazard ratio [HR] 0.56). Toxicities were modest; grade 3/4 hepatic dysfunction or decompensation, hypertension, hyponatremia, pancreatitis, weakness, diarrhea, clinical deterioration, and asthenia occurred in 1.8%–3.6% of patients.
Brivanib continues to be investigated in phase 2 and 3 trials in the first-line setting ( www.clinicaltrials.gov , NCT00355238, NCT00858871) as well as in patients with hepatic function ( www.clinicaltrials.gov , NCT00437424). Second-line studies of brivanib have yielded negative results and will be discussed under “Therapy After Progression on Sorafenib.”
Platelet derived growth factor
The PDGF family of RTKs encompasses PDGF-α, PDGF-β, FLT3, KIT, and CSF-1R. Together, PDGF and VEGF play complementary roles in angiogenesis; the former initiates the development of immature blood vessels and the latter recruits pericytes to stabilize and fuse them. The modulatory effects of PDGF on the tumor microenvironment also promote tumor progression and survival. Linifanib (ABT 869) is an oral TKI of both PDGF and VEGF signaling that is undergoing investigation in a number of malignancies including HCC. A phase 2 study reported a 4-month progression-free survival of 32% and an objective response rate of 7%. Time to radiographic progression and overall survival were 5.4 and 9.7 months, respectively. Child-Pugh A patients had better survival and response outcomes than Child-Pugh B patients. Therapy was generally well tolerated with the main side effects being diarrhea and fatigue; grade 3/4 toxicities included fatigue and hypertension that occurred in 14% and 18% of patients, respectively. One patient with Child-Pugh B liver disease died of an intracranial hemorrhage that was possibly related to linifanib. Patients who are able to tolerate extended therapy with linifanib (>48 weeks) appear to derive additional benefit in the form of disease control.
A phase 3 trial of linifanib vs. sorafenib in advanced HCC naïve to systemic therapy is currently underway ( www.clinicaltrials.gov , NCT00517920).
Multitargeted antiangiogenic TKIs
Sorafenib
Sorafenib is an orally administered multitargeted inhibitor of the VEGFR, PDGFR, Flt-3, and Raf kinases that was initially evaluated in a phase 2 trial conducted in fit patients with advanced HCC and Child-Pugh A/B liver function. Although the study was negative in regard to the primary endpoint as only 2.2% of patients had a partial response, 33.6% had stable disease for at least 4 months. This was commensurate with a median time to progression of 4.2 months and median overall survival 9.2 months, both of which compared favorably to the historical controls at the time. Grade 3 and 4 toxicities attributable to sorafenib consisted of diarrhea, fatigue, and hand–foot syndrome occurred in 5.1%–9.5% of patients. The apparent disconnect between objective response and survival benefit was highlighted also by the fact that many patients’ tumors actually increased in size. Further analysis revealed that the increase in size was accompanied by an increase in central tumor necrosis among patients benefitting from sorafenib. ,
The results of this phase 2 trial led to two multicenter phase 3 studies of sorafenib versus placebo in advanced HCC: the SHARP trial conducted mainly in the Western hemisphere and the Asia-Pacific trial. The SHARP trial enrolled 602 patients, with advanced HCC and Child-Pugh A cirrhosis. With a median time on treatment of 5.3 months, a significant improvement in median overall survival (10.7 vs. 7.9 months, HR 0.69, P < .001) was reported in favor of sorafenib. Response rates by RECIST remained almost inexistent, with 2% of patients achieving a partial response yet 71% of patients having stable disease. The most common toxicities leading to sorafenib dose reductions and/or discontinuation were fatigue, diarrhea, abdominal pain, hand–foot skin reaction, rash or desquamation, and liver dysfunction in 3%–8% of patients. Serious hepatobiliary adverse events, bleeding events, and cardiac ischemia/infarction occurred with similar frequency in both the sorafenib and placebo arms. The SHARP trial was important as the first statistically powered study to prove an overall survival benefit with sorafenib in HCC. Overall survival, calculated as the time from randomization to death, is a more definitive endpoint than progression-free survival because it is not subject to different interpretations and investigator bias.
The Asia-Pacific trial by Cheng et al. enrolled 271 patients with advanced HCC and Child-Pugh A cirrhosis in a 2:1 randomization of sorafenib versus placebo. Median overall survival again improved in favor of sorafenib (6.5 vs. 4.2 months, HR 0.68, P = .014) though only 3.3% of patients had a partial response. Reported toxicities were similar in frequency to those reported in the phase 2 and SHARP trials.
In all three trials of sorafenib, survival outcomes were significantly improved with sorafenib despite negligible response rates. These observations have led investigators to question the validity of using conventional RECIST or World Health Organization (WHO) guidelines for characterizing responses to antiangiogenic agents. In addition to the potential relevance of tumor necrosis as reported by Abou-Alfa et al., , the American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of the Liver (EASL) have proposed modified RECIST (mRECIST) guidelines which use changes in intratumoral arterial enhancement to define responses to antiangiogenic agents. In phase 2 studies of brivanib, the proportion of patients classified as clinically benefitting from therapy (i.e., objective response or disease stabilization) was consistently higher using mRECIST as opposed to modified WHO criteria, corresponding to longer progression-free survival and time to progression. , Although these disparate results are intriguing, a caveat of the mRECIST guidelines is that changes in arterial enhancement may only reflect the effects of the drug on the tumor vasculature and not actual antineoplastic activity. The ongoing debate over response definitions highlights the limitations of endpoints such as progression-free survival and time to progression that rely on radiographic criteria to define a progression “event.” In addition, because death is also considered an “event,” progression-free survival can also be influenced by factors unrelated to the study therapy or even the neoplasm itself. This is especially important in HCC where the underlying cirrhosis is as important in driving the clinical course. Validation of these novel response assessment tools against a “hard” endpoint like overall survival is needed in order to provide more definitive answers about their predictive and prognostic capabilities. In addition, an improvement in symptoms in parallel with radiographic responses would also be a valuable correlate.
Although the magnitude of benefit with sorafenib was similar between the two phase 3 trials, median overall survival was notably longer in the SHARP population compared to the Asia-Pacific population at 10.7 and 6.5 months, respectively. A head-to-head comparison of the demographic and clinical characteristics of the SHARP and Asia-Pacific populations revealed that nearly 30% more patients in the SHARP study had an ECOG PS of 0 than in the Asia-Pacific study. Moreover, more patients in the Asia-Pacific than in the SHARP group had extrahepatic metastases and BCLC stage C disease. More recently, a phase 3 trial comparing first-line sunitinib to sorafenib in advanced HCC also showed, in an exploratory analysis, that the magnitude of overall survival benefit with sorafenib compared to sunitinib was greater in non-Asians (15.1 vs. 9.3 months)) than in Asians (8.8 vs. 7.7 months). Sorafenib also significantly improved progression-free survival and time to progression in non-Asians compared to sunitinib, but there was no difference among Asians. The ethnogeographic differences in response to sorafenib may be the result of regional and cultural variations in the treatment of HCC, with Asian patients being more likely to undergo surgery or embolization for a higher disease burden than Western patients before systemic therapy is considered. The shorter survival in the Asia-Pacific study is therefore thought to be likely due to the fact that patients in Asia were generally less fit, had more advanced disease, and therefore derived less benefit from sorafenib than their Western counterparts.
A more plausible explanation for the differences in outcome that is based on the ethnicity of the patient and the etiology of the disease has surfaced as a critical one based on the results of the two studies as well as others. In the phase 2 and SHARP studies, 29%–48% and 17%–19% of patients were infected with hepatitis C and B, respectively. , In contrast, >70% of patients in the Asia-Pacific study had hepatitis B compared to only 4%–11% who had hepatitis C. A retrospective subset analysis of the phase 2 study by viral etiology reported a trend toward longer survival among patients with hepatitis C (12.4 months) than with hepatitis B (7.3 months). The beneficial effects of sorafenib were also confirmed in the subset of hepatitis C patients enrolled in the SHARP study; median overall survival was 14 months versus 7.9 months with placebo. Furthermore, in the phase 3 trial of sunitinib versus sorafenib, patients with hepatitis C had superior survival when treated with sorafenib instead of sunitinib irrespective of ethnicity (17.6 vs. 9.2 months, HR 1.52, P = .0165). There was no difference in survival between sunitinib and sorafenib among patients infected with hepatitis B. The outcomes of patients infected with hepatitis B in the SHARP trial have yet to be reported.
The differential outcomes with sorafenib in patients with hepatitis B and C are intriguing but puzzling. A potential explanation is that the hepatitis C viral core protein can activate Raf-1 kinase in HCC cells, theoretically sensitizing them to sorafenib. However, hepatitis B X protein can also activate the Raf/Mek/Erk pathway, yet the data indicate that outcomes with sorafenib are consistently better in patients who have hepatitis C. It is unlikely that changes in individual pathways can explain the differential viral–drug interactions observed; other variables including pharmacogenomics, activation of parallel pathways that enhance or counteract drug effects, among other considerations are likely at play.
Although sorafenib is well tolerated in the Child-Pugh A population, its safety in Child-Pugh B patients continues to be of concern. A subgroup analysis of the phase 2 sorafenib study reported higher rates of hepatic decompensation in Child-Pugh B compared to Child-Pugh A patients. Grade 3 and 4 hepatobiliary toxicities in the Child-Pugh A and B groups were hyperbilirubinemia (14% and 53%), encephalopathy (3% and 13%), and ascites (3% and 5%). This translated to a shorter treatment duration as well as shorter survival in Child-Pugh B (3.2 months) than in Child-Pugh A patients (9.5 months).
Similar findings have been reported from the phase 4 Global Investigation of Therapeutic Decisions in Unresectable HCC and of Its Treatment with Sorafenib (GIDEON) registry study. Compared to their Child-Pugh A counterparts, Child-Pugh B and C patients were generally less tolerant of sorafenib. Although the frequencies of drug-related toxicities was similar, Child-Pugh B and C patients were treated for a shorter duration, were more likely to develop hepatic and serious drug-related adverse events, and also experienced a lesser survival benefit on sorafenib. The study investigators suggested that patients with a score of B7/8 had outcomes comparable to those of Child-Pugh A patients, with a more distinct decrease in benefit among those with a score of B9. However, definitive conclusions cannot be drawn given that GIDEON is an uncontrolled postmarketing study that is still in progress.
Based on a phase 1 study of sorafenib pharmacokinetics in patients with hepatic dysfunction, patients with a serum bilirubin ≤1.5 × upper limit of normal (ULN) can safely initiate sorafenib at the full dose of 400 mg twice daily whereas those with a bilirubin 1.6–3 × ULN should be considered for a 200 mg twice daily dose. Sorafenib at 200 mg once daily was recommended for patients with an albumin <2.5 mg/dL and any bilirubin level. There was no safe dose identified for patients with a bilirubin >3 × ULN.
Sunitinib
Sunitinib is a multitargeted TKI of VEGFR, PDGFR, c-Kit, and Flt-3 kinases. Several phase 2 studies of sunitinib in HCC have reported modest antitumor activity; partial response rates range from 2% to 3%, 35% to 50% of patients have stable disease, median time to progression is 1.5–5.3 months, and overall survival is 8.0–9.8 months. Based on these results, a first-line phase 3 study comparing sunitinib with sorafenib in a predominantly Asian population was opened, but was terminated early because of excessive serious toxicities on the sunitinib arm. There was a nearly sixfold increase in grade 3/4 hematologic toxicities and 17% more patients had grade 3–5 bleeding events on sunitinib than sorafenib. Furthermore, 18% of patients on sunitinib died of treatment-related causes compared to 2% of patients on sorafenib. Median overall survival was inferior with sunitinib compared to sorafenib (7.9 vs. 10.2 months, HR 1.3, P = .001). Subset analyses did not reveal any ethnic, etiologic, or other subgroups showing superior outcomes with sunitinib over sorafenib.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree