Abstract
Surgery remains an integral part of the therapeutic plan for locally advanced breast cancer. Advances in systemic therapies have transformed the role of surgery for this population of patients from palliation to largely curative intent. Further, in the last decade, the integration of surgical care into the management algorithm of locally advanced breast cancer patients has also changed substantially with increasing use of neoadjuvant chemotherapy and more recently neoadjuvant endocrine therapy and targeted therapies for HER2-positive disease. Such neoadjuvant strategies have provided new opportunities to downsize the tumor burden and scope of surgical intervention. Advances in reconstructive techniques have also provided new opportunities to improve on quality of life of these heavily treated patients, although the need for radiation therapy in patients with locally advanced breast cancer remains an area of particular challenge for optimal timing of reconstruction.
Keywords
multidisciplinary care, neoadjuvant therapy, molecular assays, reconstructive surgery, breast reconstruction, chest wall reconstruction
Surgery remains an integral part of the therapeutic plan for locally advanced breast cancer. Advances in systemic therapies have transformed the role of surgery for this population of patients from palliation to largely curative intent. Further, in the last decade, the integration of surgical care into the management algorithm of locally advanced breast cancer patients has also changed substantially with increasing use of neoadjuvant chemotherapy and more recently neoadjuvant endocrine therapy and targeted therapies for HER2-positive disease. Such neoadjuvant strategies have provided new opportunities to downsize the tumor burden and scope of surgical intervention. Advances in reconstructive techniques have also provided new opportunities to improve on quality of life of these heavily treated patients, although the need for radiation therapy in patients with locally advanced breast cancer remains an area of particular challenge for optimal timing of reconstruction.
Staging System Revisions and Implications
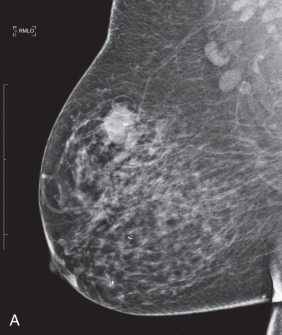
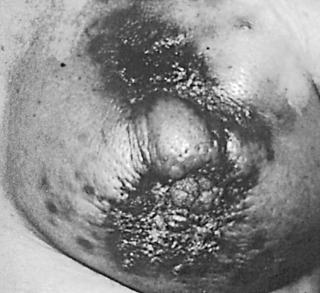
LABCs account for 10% to 15% of all newly diagnosed breast cancers, and they include tumors that are large or have extensive regional lymph node involvement and no evidence of distant metastatic spread on initial presentation. Patients with LABC have higher incidences of local and distant relapse and, concomitantly, worse survival than patients with early breast cancer. The term locally advanced breast cancer encompasses a heterogeneous group of breast neoplasms: locally recurrent (persistent) breast carcinoma, inflammatory breast carcinoma (T4d), and clinical stage IIIA, IIIB, and IIIC breast carcinomas, all of which have varying degrees and locations of lymph node involvement and some of which involve extension of cancer to the chest wall and/or skin ( Fig. 59.1 ). Inflammatory and locally recurrent carcinomas are distinct biologic entities that are discussed elsewhere in this book.
Published in 2010, the 7th edition of the American Joint Committee on Cancer (AJCC) Staging Manual builds on the extensive revisions made to the breast cancer staging system in the 6th edition and reflects knowledge gained from improved and evolving application of sentinel lymph node biopsy, immunohistochemistry, molecular techniques, improved imaging modalities including magnetic resonance imaging (MRI), and the results of clinical trials. With regard to nodal staging, classification of isolated tumor cell clusters and single cells is now more stringent: small clusters of cells not greater than 0.2 mm and nonconfluent or nearly confluent clusters of no more than 200 cells in a single histologic lymph node cross section are classified as isolated tumor cells, that is, pN0 (i+). Likewise, in patients who have received neoadjuvant systemic therapy, posttreatment nodal metastases no greater than 0.2 mm are classified as ypN0 (i+), and these patients are not considered to have achieved a pathologic complete response (pCR). Furthermore, documentation of posttreatment response to neoadjuvant therapy must also describe the modality—physical examination, imaging (mammogram, ultrasound, MRI), or pathology (fine-needle aspiration [FNA], core needle biopsy, sentinel lymph node biopsy)—through which response to treatment is assessed. Finally, it has been clarified that the sentinel node modifier (sn) should be omitted if six or more sentinel nodes are identified on gross examination of surgical pathology specimens. Given the prognostic significance of nodal disease burden in breast cancer, the increasingly refined assessment of both nodal involvement and clinical significance enabled by recent clinical trials and reflected in these staging guidelines has allowed clinicians to provide more multimodal and often less invasive treatment for patients presenting with LABC. The 8th edition of the AJCC staging system contains biological factors in addition to the anatomic designations of tumor (T), nodal (N), and metastasis (M).
Pretreatment Evaluation, Diagnosis, and Management
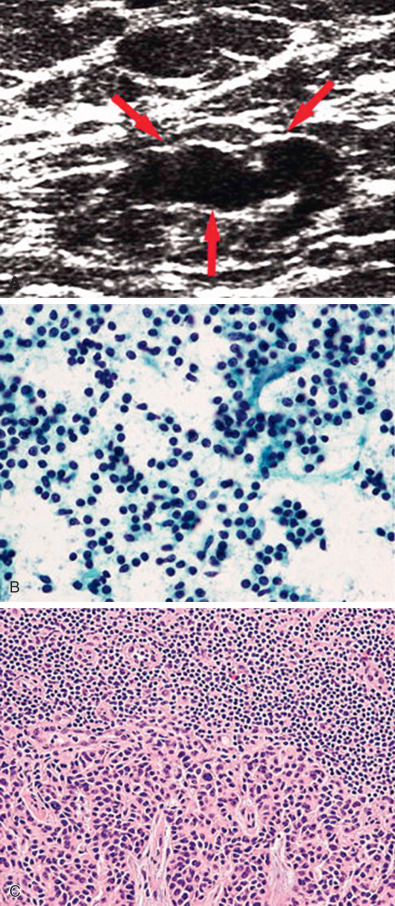
Establishing a tissue diagnosis is a priority for patients presenting with LABC. After thorough breast imaging, which includes bilateral diagnostic mammography and ultrasonography of the breast and nodal basins, core needle biopsy of the primary tumor should be performed to provide tissue for histopathologic examination and determination of hormone receptor status and HER2/neu expression. If breast conserving surgery (BCS) is being considered, a clip should be placed into the primary tumor site before initiation of any systemic therapy. Matted, fixed, or sonographically suspicious axillary, supraclavicular, infraclavicular, and even internal mammary lymph nodes should be subjected to FNA biopsy for more definitive staging by radiologists with the expertise to perform this procedure accurately and safely. Core needle biopsy can also be performed and may be preferred when cytologic expertise is not available ( Fig. 59.2 ).
A complete staging workup includes a thorough history and physical examination, a complete blood cell count with differential and platelet counts, a biochemical survey (i.e., comprehensive metabolic panel including electrolytes and liver enzymes), chest radiography, imaging for distant disease including a bone scan and abdominal cross-sectional imaging. Patients with bone pain, abnormal results on a bone scan, or elevated alkaline phosphatase levels should have pertinent bone radiographs obtained to rule out osseous metastases. Each patient should be evaluated in a multidisciplinary context with a team including surgical, medical, and radiation oncologists; radiologists; pathologists; and plastic surgeons. A consensus treatment plan should be presented to the patient initially and should be reviewed throughout the course of treatment in the context of response to neoadjuvant systemic therapy as well as postoperatively to review pathologic analysis of the surgical specimen.
Unimodal Treatment Approaches
As with early breast cancer, the most important outcomes used to assess treatment efficacy in patients with LABC are locoregional control and survival.
Surgery
Surgery is the oldest treatment for breast cancer, but enthusiasm for radical surgical resections has waxed and waned over the years. At the end of the 19th century, William Halsted described the radical mastectomy, which involved removal of the entire breast with en bloc removal of all axillary lymphatics, the chest wall musculature, and part of the sternum and ribs if they were involved with tumor. Despite this aggressive approach to locally advanced tumors, survival remained poor, ranging from 13% to 20% at 5 years.
Awareness of variations in biology (and therefore the effectiveness of therapy) of LABC stems from Haagensen and Stout’s pioneering insights derived from nearly 30 years of cumulative experience in breast cancer management. On the basis of a review of 1135 breast cancer patients treated with radical mastectomy at Presbyterian Hospital in New York from 1915 to 1942, these authors observed that patients with certain features of LABC were beyond cure, even with radical surgery. Haagensen’s “grave signs” included edema of the skin of the breast, skin ulceration, chest wall fixation, an axillary lymph node greater than 2.5 cm in diameter, and fixed axillary nodes. Patients with two or more of these signs had a 42% local recurrence rate and a 5-year disease-free survival rate of only 2%.
McWhirter and colleagues demonstrated that less disfiguring surgery produced results similar to those seen with the more aggressive radical mastectomy, an idea that led to the recognition that treatment failure from breast cancer stemmed from systemic dissemination before surgery. The Alabama Breast Project examined the efficacy of modified radical mastectomy versus radical mastectomy as unimodal therapy for stage III breast carcinoma in patients with a minimum of 10 years’ follow-up. In this study, patients with stage III disease who underwent modified radical mastectomy represented only 6% of the total study population but had 20% of the local recurrences. In contrast, patients with stage III disease who underwent radical mastectomy represented 5% of the total study population but had 6% of the local recurrences. Radical mastectomy significantly decreased the rate of local recurrence and improved survival compared with modified mastectomy, albeit with greater morbidity. The Alabama experience further supported the importance of exploring whether multimodal therapy might yield survival rates similar to those of radical mastectomy without the severely morbid sequelae of radical surgical interventions.
Radiotherapy
After Haagensen and Stout’s publication of the criteria for inoperability, questions arose in the early 20th century surrounding the concept of radical en bloc ablation and whether radiation could be substituted for, or at least improve the results of, radical surgery. In 1949 Baclesse used radiotherapy alone to achieve local tumor control in select patients with advanced breast cancer. In 1965, Fletcher and Montague from the University of Texas MD Anderson Cancer Center reported a 70% local control rate for advanced breast cancer treated with radiotherapy alone. Distant metastasis occurred an average of 18 months later, and the 5-year overall survival rate was 25%. A 1976 retrospective review of the Joint Center for Radiation Therapy experience by Harris and associates reported a 54% rate of 5-year local tumor control and a 30% rate of 5-year overall survival using radical radiotherapy (dose >60 Gy) alone. The National Cancer Institute in Milan, Italy, published its experience with “supervoltage” radiotherapy in the treatment of LABC in 1973. This retrospective study reported an encouraging 50% control rate. However, 45% of the patients with “controlled” disease experienced relapse within 18 months of beginning radiotherapy, and 82% of those relapses occurred at a distant site. Overall, a 21% 5-year survival rate was achieved. However, the “supervoltage” radiotherapy, which included doses of 80 to 90 Gy, was not benign: fibrosis, skin ulceration, chest wall necrosis, pathologic fractures, cardiac and pulmonary complications, brachial plexus injury, and severe lymphedema of the ipsilateral arm were common and debilitating occurrences. In summary, early studies served to demonstrate that radiotherapy may be better suited as a component of the multimodal treatment algorithm for LABC rather than as monotherapy due to its dose-limiting side effects.
Multimodal Approaches
Experiences with unimodal therapy demonstrated that good local control rates achieved with surgery or radiotherapy alone did not correlate with good prognosis and long-term survival because hematogenous metastases were not being controlled. Consequently, by the late 1970s, systemic chemotherapy was an integral part of the primary management of LABC, a development that occurred in large part as a result of several major prospective multimodal trials including the National Cancer Institute (Milan, Italy) trials in 1973 and 1975 and an MD Anderson Cancer Center trial in 1974.
Early Trials
In the first Milan trial, patients with stage IIIA or IIIB breast cancer were given four cycles of doxorubicin and vincristine, followed by 60 Gy to the breast and a 10-Gy boost to the area of residual tumor. Patients who demonstrated a complete response were randomized to either no further treatment or six more courses of chemotherapy. The objective response rate to this neoadjuvant chemotherapy regimen was 89%, the complete response rate was 15.5%, the major (≥50% tumor size reduction) response rate was 54.5%, and the minor (<50% tumor size reduction) response rate was 19%. Of the patients responding to neoadjuvant chemotherapy, 83% had a complete response with the addition of radiotherapy. This combined chemotherapy-radiotherapy approach resulted in an overall 3-year survival rate of 53%.
In 1975, the Milan group began a second trial that ultimately enrolled 277 consecutive patients with stage IIIA and IIIB disease. In this trial, patients received three courses of doxorubicin and vincristine preoperatively. They were randomized to radiotherapy or surgery (radical or modified radical mastectomy) followed by six additional cycles of chemotherapy. The best local control was achieved when surgery rather than radiotherapy was interposed between chemotherapy courses (82.3% vs. 63.9% complete local control rate). Freedom from disease progression was maintained for 5 years or longer in 25% of the patients who received chemotherapy and surgery but in only 4.9% of the patients who received chemotherapy and radiotherapy. Likewise, the overall 5-year survival rate was higher for the chemotherapy and surgery group (49.4% vs. 19.7%).
In 1974, a multimodality treatment protocol for LABC was initiated at MD Anderson Cancer Center to explore whether the combined use of chemotherapy, surgery, and radiotherapy would improve control of micrometastases and reduce local tumor burden in patients with stage III disease, thereby avoiding the need for either radical radiotherapy or radical mastectomy, both of which were standard of care for stage III patients at that time. Between 1974 and 1985, 174 patients with stage III noninflammatory (operable and inoperable) breast cancer were initially treated for three cycles with combination systemic therapy consisting of 5-fluorouracil, doxorubicin (Adriamycin), and cyclophosphamide (Cytoxan) (FAC); up until 1978, bacillus Calmette-Guerin (BCG) was also included in this regimen.
After three cycles of neoadjuvant chemotherapy, patient response was assessed with a combination of clinical examination and mammography, and patients were assigned to one of three treatment arms based on clinical response: (1) those who had minimal or no residual disease (i.e., complete responders ) were assigned to radiotherapy only (although it is important to note that in the early years of the trial, complete responders with large breasts sometimes underwent postchemotherapy, preradiation mastectomy to facilitate delivery of radiation); (2) those who had a moderate response (i.e., moderate responders ) went on to modified radical mastectomy followed by radiotherapy (beginning in 1978, moderate responders also received adjuvant chemotherapy, the regimens for which varied over time, after undergoing surgery and before receiving radiation); and (3) those who had no response or progressive disease (i.e., nonresponders ) went on to radiotherapy, with subsequent surgical resection if their disease was operable. In addition, although surgery alone was not a predetermined treatment arm, a subset of 40 patients underwent surgical resection alone for a variety of reasons including patient preference, patient comorbidities that precluded radiation, development of distant disease before receiving radiation, and provider preference or judgment.
With a median follow-up of 59 months, complete remission was achieved in 16.7% of patients and was more common in stage IIIA patients than in stage IIIB patients (17% vs. 8%); 70.7% of patients had a moderate response after the initial three cycles of neoadjuvant chemotherapy, again with higher rates of response among stage IIIA patients. All but 6 of the 174 treated patients were eventually rendered disease-free after neoadjuvant chemotherapy and local treatment; all 6 of the patients with residual/progressive disease had stage IIIB disease at presentation. Five-year overall and disease-free survival rates for stage IIIA patients were both 84%, whereas for stage IIIB patients, overall and disease-free survival rates were 44% and 33%, respectively. These findings demonstrated significant improvement over historical 5-year survival rates of 30% to 45% for stage IIIA and only 10% to 28% for stage IIIB patients and illustrated the efficacy of a multimodal approach in which systemic therapy mitigated the need for radical local therapy.
Chemotherapy
Neoadjuvant chemotherapy is an important component in the management of LABC. Although randomized trials have demonstrated that neoadjuvant chemotherapy and adjuvant chemotherapy are associated with similar recurrence-free and overall survival rates when the same regimen is applied, neoadjuvant treatment nevertheless has many advantages over adjuvant therapy with regards to LABC.
Neoadjuvant chemotherapy can (1) convert LABC from inoperable to operable, (2) increase the rate of BCS, (3) serve as an in vivo chemosensitivity test for a given tumor, and (4) allow treatment of micrometastatic disease at the earliest possible opportunity. Pathologic response to neoadjuvant systemic therapy in the breast and lymph nodes correlates with patient survival. A potential disadvantage rests in the loss of prognostic information provided by tumor size and nodal status in an untreated surgical specimen, but it has been shown that patients who have a complete clinical or pathologic response with neoadjuvant chemotherapy have improved overall and disease-free survival. Multiple, large randomized trials have proven the safety of neoadjuvant chemotherapy in LABC and have shown objective response rates ranging from 60% to 80% as well as less morbidity than is observed with adjuvant chemotherapy.
The response of patients to neoadjuvant chemotherapy in patients with LABC depends in large part on the agents or combination of agents used ( Box 59.1 ). Randomized trials have confirmed the superiority of anthracycline-based regimens over cyclophosphamide, methotrexate, and 5-fluorouracil in the treatment of early, locally advanced, and metastatic breast cancer. The rates of pCR to neoadjuvant systemic therapy vary according to treatment regimen, ranging from 6% to 15% with anthracycline-based regimens to almost 30% with the addition of a taxane ( Table 59.1 ). The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-27 trial randomized patients with resectable breast cancer into one of three neoadjuvant treatment arms: doxorubicin plus cyclophosphamide, doxorubicin plus cyclophosphamide and docetaxel, or preoperative doxorubicin and cyclophosphamide followed by postoperative docetaxel. The investigators observed a pCR rate of 26% associated with the addition of docetaxel to the preoperative anthracycline regimen.
Regimens for HER2-Nonamplified Disease
- •
Paclitaxel × 12 weekly cycles followed by AC × 4 cycles (administered either as dose-dense every 2 weeks w/G-CSF support or every 3 weeks)
- •
TC × 4 cycles (administered every 3 weeks) with G-CSF support
- •
- •
Regimens for HER2-Amplified Disease
- •
AC × 4 cycles (administered every 3 weeks) followed by THP × 4 cycles (continuation of trastuzumab to complete 1 year of therapy)
- •
TCHP × 6 cycles (administered every 3 weeks with continuation of trastuzumab to complete 1 year of therapy)
AC, Adriamycin (doxorubicin) 60 mg/m 2 IV + Cytoxan (cyclophosphamide) 600 mg/m 2 IV; G-CSF, Granulocyte colony stimulating factor; IV, intravenous; Paclitaxel, 80 mg/m 2 IV weekly; TC, Taxotere (docetaxel) 75 mg/m 2 IV + Cytoxan (cyclophosphamide) 600 mg/m 2 IV; TCHP, Taxotere (docetaxel) 75 mg/m 2 IV + carboplatin AUC (area under the curve) 6 mg/mL IV + Herceptin (trastuzumab) 8 mg/kg IV loading followed by 6 mg/kg IV maintenance + Perjeta (pertuzumab) 840 mg IV loading followed by 420 mg IV maintenance; THP, Taxotere (docetaxel) 75 mg/m 2 IV + Herceptin (trastuzumab) 8 mg/kg IV loading followed by 6 mg/kg IV maintenance + Perjeta (pertuzumab) 840 mg IV loading followed by 420 mg IV maintenance.
| Trial | Population | Regimen: pCR Rate | Comments |
|---|---|---|---|
| NSABP B-27 | 802 patients 31% clinically node positive (N1), T1c–T3 | AC → T: 63% | |
| TRYPHAENA | 225 patients Locally advanced/operable or inflammatory HER2+ breast cancer | TCHP × 6 cycles (n = 76): 47.5% in the HR+ subgroup (and 81.1% in the HR− subgroup FEC × 3 cycles → THP × 3 cycles (n = 75): 45.7% in the HR+ subgroup, 62.5% in the HR− subgroup | |
| NeoSphere | 417 patients Locally advanced/operative or inflammatory HER2+ breast cancer, primary tumor >2 cm | THP × 4 cycles (n = 107): 39.3% (treated with FEC × 3 cycles postoperatively) | |
| GEPARTRIO | 2090 patients with untreated breast cancer, of which 1390 responders randomized to receive four or six additional cycles | TAC × 8 cycles (n = 704) with clinical response of >50% reduction after initial two cycles): 23.5% TAC × 6 cycles (n = 686, with clinical response <50% after initial two cycles): 21% |
|
A retrospective study in Nottingham, England, compared outcomes in 106 consecutive patients with LABC who received one of two neoadjuvant chemotherapy regimens: an anthracycline-based regimen (5-fluorouracil, epirubicin/Adriamycin, cyclophosphamide; i.e., FEC/FAC) or a regimen consisting of mitoxantrone, methotrexate, and mitomycin (MMM). End points of locoregional recurrence, metastasis, and survival were analyzed after a median follow-up of 54 months. All patients underwent neoadjuvant chemotherapy as part of a multimodal approach, which included subsequent mastectomy, radiotherapy, and adjuvant endocrine therapy if tumors were estrogen receptor (ER) positive. More patients in the anthracycline-based treatment group than in the MMM group had a complete clinical response (24% vs. 9%, p = .035). In addition, patients in the anthracycline group had a lower incidence of locoregional recurrence (6% vs. 19%) and distant metastasis (20% vs. 53%) and a higher survival rate (82% vs. 45%), findings that supported the use of anthracycline-based neoadjuvant regimens in LABC.
Although the effectiveness of anthracyclines and taxanes in treating breast cancer has now been demonstrated in multiple trials, these agents are associated with significant morbidity including but not limited to neurologic and cardiac toxicities. Thus increasing attention has been devoted to maximizing cure at the population level while preventing overtreatment of the individual through a shift in focus from standardized chemotherapy treatment protocols to patient-specific treatments based on gene-expression signatures. This personalized approach to systemic therapy has been furthered greatly by the development and clinical use of commercially available genomic assays such as Onco type DX, MammaPrint, Mammostrat, and Prosigna, which were developed to assess the appropriateness of chemotherapy in the adjuvant setting in patients with early-stage breast cancer whose initial presentation did not suggest a need for chemotherapy. With increasing confidence in these genomic assays to tailor systemic therapy decisions among early-stage breast cancer patients, these assays are increasingly being tested for utility in tailoring treatment in patients with more advanced disease, in particular in those LABC patients with the ER-positive, HER2/neu nonamplified disease.
Developed in 2004, Onco type DX (Genomic Health, Redwood, CA) is a 21-gene recurrence score assay that has been validated in women receiving adjuvant tamoxifen with ER-positive, HER2/neu nonamplified (HER2-negative), node-negative breast cancer as being able to quantify both the likelihood of distant recurrence within 10 years (i.e., is prognostic ) and also the likely magnitude of improved distant-recurrence-free survival that would occur with receipt of adjuvant hormonal and chemotherapy as opposed to only receiving hormonal therapy (i.e., is predictive ).
Onco type DX uses formalin-fixed, paraffin-embedded (FFPE) samples from surgical specimens to categorize patients into one of three groups based on recurrence scores—low (<18), intermediate (18–30), and high (≥31)—reflecting their likelihood of distant recurrence in 10 years. In the Trial Assigning IndividuaLized Options for Treatment (Rx), or TAILORx, women with a low recurrence score were found to have a less than 1% risk of recurrence in 10 years with receipt of endocrine therapy alone, thereby supporting a shift toward increasingly selective prescription of chemotherapy within the context of multimodal treatment for patients with ER-positive, HER2-negative disease. The applicability of Onco type DX for prognosis for patients with more advanced disease is currently under investigation: the RxPONDER Trial (Rx for Positive Node, Endocrine Responsive Breast Cancer) was initiated in 2011 and is designed to determine whether ER-positive, HER2-negative patients with one to three involved axillary lymph nodes and low to intermediate Onco type DX scores would benefit from adjuvant chemotherapy and endocrine therapy versus adjuvant endocrine therapy alone. Another aim of this trial is to determine whether there is an optimal recurrence score cutoff point for these patients, above which chemotherapy should always be recommended. Other genomic assays are the subject of ongoing investigation and validation in patients with invasive disease with varying combinations of biomarkers (e.g., Neoadjuvant Breast Registry Symphony Trial [NBRST]). The long-term results of these trials are eagerly awaited and hold great promise for the future of personalized management of breast cancer.
Endocrine Therapy
Endocrine therapy is a critical component of multimodal care for patients with ER-positive breast cancer. In a phase III trial launched by the European Organization for Research and Treatment of Cancer (EORTC), 410 patients with LABC were randomized to receive radiotherapy alone, radiotherapy plus chemotherapy, radiotherapy plus endocrine therapy, or radiotherapy plus endocrine therapy and chemotherapy. Endocrine therapy consisted of ovarian irradiation for premenopausal women and tamoxifen 10 mg twice daily for 5 years for postmenopausal women. After an 8-year follow-up, the combination of adjuvant chemotherapy with endocrine therapy produced a significant reduction in the risk of locoregional recurrence (from 60%–47%) and distant progression of disease. Although the combined treatments provided the greatest therapeutic effect, patients who received adjuvant endocrine therapy appreciated a significant improvement in survival with a 25% reduction in the death hazard ratio. Thus, current recommendations dictate that premenopausal patients with hormone receptor–positive breast cancer receive at least 5 years of adjuvant tamoxifen, whereas postmenopausal women should receive an aromatase inhibitor, unless otherwise contraindicated based on each agent’s described risk profile (discussed elsewhere in this book). It is important that the clinical team factor patient and tumor-specific features factor into the complex multimodal treatment equation.
In addition to tamoxifen and aromatase inhibitors, antiestrogen therapies including fulvestrant (which blocks ER and promotes its degradation) and aromatase inhibitors in combination with other agents (e.g., letrozole and palbociclib, a selective inhibitor of cyclin-dependent kinases [CDKs] 4 and 6) have been shown to improve progression-free survival in advanced breast cancer and are used in treatment of locally recurrent and/or treatment-resistant, hormone-sensitive cancers. Furthermore, pharmacologic suppression of ovarian function has also proven to be an important component of endocrine therapy: results published in 2015 from the Suppression of Ovarian Function Trial (SOFT) trial demonstrated that in young women with ER-positive breast cancer who remained premenopausal (determined by estradiol levels) after receiving chemotherapy, ovarian suppression with triptorelin, a gonadotropin-releasing hormone (GnRH) agonist, given in combination with tamoxifen or with an aromatase inhibitor reduced the risk of recurrent breast cancer compared with tamoxifen alone.
Primary endocrine therapy is increasingly used in the neoadjuvant setting and has been demonstrated to result in significant increases in breast conservation rates and improved postsurgical outcomes in patients with stage II and III ER-positive breast cancer. Pathologic complete response rates are low compared with those observed with systemic chemotherapy; however, the Preoperative Endocrine Prognostic Index (PEPI score; ki67 proliferation biomarker, tumor size, nodal status and ER status) is a reliable predictor of prognosis after treatment with endocrine therapy. Three to 4 months of therapy are typically administered and then treatment response is analyzed. In a phase III randomized trial in postmenopausal women, aromatase inhibitors, such as letrozole, had better efficacy compared with tamoxifen, and the phase II American College of Surgeons Oncology Group (ACOSOG) Z1031 trial also demonstrated the efficacy of anastrozole, letrozole, and exemestane in the neoadjuvant setting for postmenopausal patients with ER-positive disease.
The ALTERNATE (ALTernate approaches for clinical stage II or III Estrogen Receptor positive breast cancer NeoAdjuvant TrEatment in postmenopausal women) trial is an ongoing phase III study launched by the Alliance for Clinical Trials in Oncology in which postmenopausal women with clinical T2–4, node-positive, ER-positive, HER2-negative breast cancer are randomized to three endocrine therapies—(1) the ER downregulator fulvestrant, (2) the aromatase inhibitor anastrozole, and (3) the combination of fulvestrant and anastrozole—to help characterize the patients with hormone-responsive disease for whom adjuvant chemotherapy may be omitted and also to identify clinicopathologic characteristics of ER-positive, endocrine-resistant tumors that can be targeted in future therapeutic investigation.
Targeted Therapy
The development of anti-HER2 targeted therapy has transformed HER2/neu amplified (HER2-positive) breast cancer from a disease with a poor prognosis to an opportunity for improved survival and cure. Multiple trials have now demonstrated improved rates of BCS, pCR, and survival when neoadjuvant trastuzumab is administered in conjunction with standard chemotherapy regimens. Lapatinib has also been found to be effective in the treatment of HER2-positive breast cancer but has side effects that are generally found to be more severe than trastuzumab. More recently, pertuzumab has emerged as a powerful adjunct to trastuzumab and docetaxel in the treatment of HER2-positive breast cancer, initially in the setting of demonstrably improved progression-free survival in patients with metastatic disease via the CLEOPATRA trial. TRYPHAENA and NEOSPHERE are both randomized phase II trials that have demonstrated the efficacy of pertuzumab in improving rates of pCR in HER2-positive LABC without unacceptable cardiac toxicity when administered in conjunction with trastuzumab and docetaxel. The multinational phase III APHINITY trial will test whether the combination of pertuzumab and trastuzumab will result in improved disease-free survival in the adjuvant setting compared with single-agent adjuvant therapy with trastuzumab.
Adjuvant Radiotherapy
Postmastectomy radiotherapy (PMRT) as a component of multimodal therapy is considered standard of care for LABC patients. The 2016 National Comprehensive Cancer Network breast cancer guidelines recommend PMRT for patients with four or more positive lymph nodes. In addition, the guidelines urge practitioners to “strongly consider” PMRT in patients with one to three positive nodes and to “consider” PMRT in select patients with node-negative disease but with clinical features that put them at increased risk for local recurrence including tumor size 5 cm or greater and tumor size less than 5 cm but with less than 1 mm margins. Most patients with LABC receive neoadjuvant chemotherapy, and it remains to be determined whether PMRT is beneficial in patients with LABC who experience pCR after neoadjuvant chemotherapy. A 2002 study at MD Anderson Cancer Center noted that the presenting stage of the disease is the most important predictor of locoregional recurrence and should factor into decision-making regarding adjuvant radiotherapy, even in patients who have a complete pathologic response to neoadjuvant chemotherapy. NSABP B-51 is an ongoing phase III, randomized trial, the results of which will help provide more information about the most appropriate role for radiotherapy in the management of LABC after receipt of neoadjuvant chemotherapy, particularly in patients who experience pCR.
In 2015, results from two randomized trials, the MA.20 study from Canada and an EORTC trial on nonaxillary, regional nodal irradiation, demonstrated that regional nodal irradiation could significantly improve outcomes for patients with LABC. In the MA.20 trial, 1832 women with node-positive (clinical N1 only) or node-negative, high-risk (i.e., T3 tumors; T2 tumors with <10 lymph nodes removed in axillary lymph node dissection and one or more factors of ER-negative status, grade 3 histology, or lymphovascular invasion) breast cancer were randomized from 2000 to 2007 to receive either whole breast irradiation only (i.e., the control group) or whole breast irradiation in conjunction with irradiation of the axillary, internal mammary, and supraclavicular lymph node basins. There was no difference between the two treatment arms in 10-year overall survival rates, but the group with nodal irradiation had higher disease-free survival (82% vs. 77%, p = .01) at 10 years as well as higher rates of grade 2 or higher acute pneumonitis (8.4% vs. 4.5%, p = .001). Notably, the improved disease-free survival seen in the group with nodal irradiation was in large part due to decreased recurrence in the lymph nodes, not decreased in-breast recurrence.
EORTC 22922/10925 was a phase III trial designed specifically to evaluate the potential benefit of adding internal mammary and medial supraclavicular radiation to the treatment of patients with central/medial breast cancers or with outer quadrant breast cancer and axillary involvement because both these sets of patients are at high risk for harboring microscopic extraaxillary regional disease. Between 1996 and 2004, 4004 patients, all of whom underwent axillary lymph node dissection, were randomized to receive either whole breast/chest wall irradiation only (i.e., the control group) or whole breast/chest wall irradiation with irradiation of the internal mammary and medial supraclavicular lymph node basins. At 10 years, the group with nodal irradiation had higher disease-free survival (72.1% vs. 69.1%, p = .04) and higher distant disease-free survival (78% vs. 75%, p = .02) as well as lower breast cancer–specific mortality (12.5% vs. 14.4%, p = .02), but there was no statistically significant difference between the two groups with regards to 10-year overall survival (82.3% vs. 80.7%, p = .06). The nodal irradiation group also had higher rates of pulmonary fibrosis. Thus these two trials demonstrated that nodal irradiation decreases the risk of locoregional—and perhaps even distant—recurrence but not without some additional morbidity.
Breast Conserving Surgery in Locally Advanced Breast Cancer
Although modified radical mastectomy has historically been the standard of care for LABC, BCS after neoadjuvant chemotherapy is increasingly performed for LABC in patients whose disease responds to systemic therapy. An early study from MD Anderson Cancer Center retrospectively analyzed surgical specimens from patients with stage IIIA and IIIB breast cancer after three cycles of neoadjuvant cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy, citing complete and partial pathologic response rates of 16% and 84%, respectively. On the basis of criteria for breast conservation in early-stage breast cancer, they concluded that 23% of those patients could have undergone BCS after their neoadjuvant treatment. Schwartz and colleagues retrospectively analyzed patients with stage IIB and III breast cancer who received induction chemotherapy and reported 5-year disease-free and overall survival rates of 56% and 67% for patients treated with mastectomy versus 77% and 80%, respectively, for patients treated with BCS; when patients were stratified according to age and degree of pathologic response; however, no difference in survival was seen between the two arms. A 2004 report of the MD Anderson experience by Chen and associates analyzed locoregional recurrence rates in 130 women with stage IIIA–C breast cancer who underwent neoadjuvant chemotherapy and subsequent BCS. In-breast recurrence-free and locoregional recurrence-free survival rates of 95% and 91%, respectively, were reported at 5 years, demonstrating that BCS after neoadjuvant chemotherapy results in acceptably low recurrence rates in patients with LABC while citing the presence of N2 to N3 disease, residual tumor greater than 2 cm in size, multifocal disease, and lymphovascular invasion as predictors of increased recurrence. Thus BCS is an acceptable option in terms of local control and survival for patients with LABC who experience a significant treatment response to neoadjuvant chemotherapy, and this option is offered to appropriate patients at our institution ( Box 59.2 ).
Advanced Breast Cancer
- •
Resolution of skin edema (dermal lymphatic involvement)
- •
Residual tumor size for which resection would not render a cosmetically unacceptable postoperative appearance of the breast
- •
Absence of extensive intramammary lymphatic invasion
- •
Absence of extensive suspicious-appearing microcalcifications
- •
No known evidence of multicentric disease
- •
Patient’s desire for breast preservation
Axillary Staging
Axillary Lymphadenectomy
Rationale
Level I and II axillary lymph node dissection (ALND) remains the standard of care in LABC, particularly in cases of biopsy proven axillary nodal metastases. ALND likely contributes little to overall survival but is crucial for staging and prognostic information as well as regional control.
Level I lymph nodes are located lateral to the pectoralis minor muscle, level II lymph nodes are located posterior to the pectoralis minor muscle. Level III lymph nodes are located medial to the pectoralis minor muscle and are not routinely dissected in breast cancer patients. Rotter nodes are located between the pectoralis major and minor muscles. As a rule, radiation therapy to the axilla is not used in conjunction with complete (level I/II with or without level III) ALND. In addition, the level I/II dissection avoids the functional and cosmetic difficulties that can result from axillary radiotherapy in an axilla that has already been completely dissected. Because moderate doses of radiation therapy sterilize lymph nodes 1 cm or smaller, metastatic cancer in smaller, centrally located axillary lymph nodes (high level II and level III) are generally sterilized with radiation therapy. With use of such a combination of therapeutic modalities, the entire axilla is treated with minimal overlap of radiation portals with the surgically dissected portion of the axilla.
Technique
The technique of ALND is the same regardless of whether the patient is undergoing the procedure at the time of modified radical mastectomy or through a separate incision in cases of BCS. The technique of modified radical mastectomy is described elsewhere in this text. When ALND is performed as part of a modified radical mastectomy, the mastectomy incision is oriented so as to include the tumor mass, all involved skin, the nipple-areola complex, and previous biopsy sites as well as to allow easy access to the axilla. The arm is draped free so that the elbow may be flexed or extended and the arm adducted across the chest wall. Skin flaps for axillary dissection are developed superiorly to the level of the clavicle, medially to the medial half of the clavicle, and laterally to the deltopectoral triangle and cephalic vein. The lateral portion of the superior flap is developed until the anterior edge of the latissimus dorsi muscle is identified, at which point the superior flap is developed laterally and superiorly following the anterior edge of the latissimus dorsi muscle cephalad ( Fig. 59.3 ). As the dissection continues toward the axilla, the major branch of the intercostobrachial nerve is identified. This nerve is composed of fibers from the lateral cutaneous branches of the second and third intercostal nerves and runs at right angles and anterior to the latissimus dorsi muscle. It can be sacrificed if multiple clinically positive lymph nodes are present, with the postoperative sequelae being paresthesias to the medial upper arm. The dissection is continued cephalad following the anterior border of the latissimus dorsi muscle until the white tendon of the latissimus dorsi muscle is identified. Immediately superior and anterior to this tendon, the axillary vein is identified and exposed.
Attention is then turned to dissection of the inferior flap. In elevating the skin flaps medially, the plane of penetration of the pectoralis major fascia by the perforating branches of the internal mammary vessel should be used as a guide for the medial extension of the dissection. The breast is dissected free from the pectoralis major muscle, beginning superiorly and medially and progressing inferiorly and laterally until the lateral border of the muscle is identified almost in its entirety.
The lateral border of the pectoralis major muscle is then retracted inferiorly and medially. Dissection is continued lateral and posterior to the pectoralis major muscle to identify the lateral border of the pectoralis minor muscle. The entire innervations of the pectoralis major and minor muscles can be preserved in this operation. The medial pectoral nerve is identified coursing lateral to (or penetrating) the pectoralis minor muscle at approximately the juncture between the superior one-third and inferior two-thirds of the pectoralis major muscle. The clavipectoral fascia lateral to the pectoralis minor muscle is opened, and the axillary vein is again identified. Working laterally along the axillary vein, the venous tributaries coursing inferiorly are divided and ligated. Laterally, the neurovascular bundle to the latissimus dorsi muscle is identified; this bundle contains the thoracodorsal nerve and major branches of the subscapular artery and vein. The lateral thoracic artery is usually identified just medial to it and is removed with the specimen. At the site of confluence of the thoracodorsal neurovascular trunk with the latissimus dorsi muscle, a venous tributary courses medially to join the chest wall. At this site, with meticulous dissection in a plane parallel to the long axis of the patient, the long thoracic nerve to the serratus anterior muscle (respiratory nerve of Bell) is best identified. The long thoracic nerve is traced superiorly until it exits the operative field posterior to the axillary vein.
The axillary contents are then removed from the serratus anterior muscle anterior and medial to the long thoracic nerve. The superior extent of the axillary dissection was previously defined by the axillary vein, and the medial extent of the dissection is represented by the lateral borders of the pectoralis major and minor muscles as well as the medial pectoral nerve and accompanying vascular structures. During surgery on a patient with stage IIIA, stage IIIB, or inflammatory (T4d) breast cancer who is to receive postoperative radiation therapy, any lymph nodes that are clinically positive should be removed from beneath the pectoralis minor muscle (within level II lymph nodes). The specimen is removed from the operative field and oriented for the pathologist, the wound is irrigated copiously with warm saline, and a closed-suction Silastic drain is placed in the dissected axillary space. The drain is sutured in place at the skin entrance site, and the wound is closed in two layers.
Role of Sentinel Lymph Node Biopsy
Patients with LABC routinely receive neoadjuvant chemotherapy as part of their multimodal treatment, and 30% to 40% of patients with LABC experience pCR in the nodal basin after neoadjuvant chemotherapy, with anti-HER2 therapy achieving even higher rates of pCR (as discussed earlier). The accuracy of sentinel lymph node biopsy (SLNB) in this patient subset has been the subject of significant investigation, especially with regard to whether ALND can be omitted in patients who have clinical evidence of nodal response that is confirmed by a negative SLNB ( Table 59.2 ).
| ACOSOG Z1071 (n = 637) | SENTINA (Arm C) | SN FNAC | |
|---|---|---|---|
| Nodal eligibility criteria | cN1–2 | cN1–2 | cN1–2 |
| SLN identification rate | 92.7% | 87.8% | 87.6% |
| Overall FNR (no IHC) | 12.6% | 14.2% | 13.4% |
| FNR depending on Mapping agents | |||
| One agent | 20.3% | 16% | 16% |
| Dual agent | 10.8% | 8.6% | 5.2% |
| FNR by number of SLNs | |||
| 1 SLN 2 SLNs ≥3 SLNs | 31% 21.1% 9.1% | 24.3% 18.5% 4.9% | 18.2% ≥2 SLNs = 4.9% |
| FNR with IHC | 8.7% | Not reported | 8.4% |
The ACOSOG Z1071 trial was launched in 2009 to investigate the false-negative rate of SLNB (with at least two nodes removed) after neoadjuvant chemotherapy in breast cancer patients with pretreatment nodal disease confirmed on needle biopsy; an acceptable false-negative rate was predetermined to be 10%. Of 756 patients enrolled in the trial, 649 with clinical N1 (cN1) disease and 38 with clinical N2 (cN2) disease ultimately underwent SLNB and ALND after completing chemotherapy; 2 patients underwent SLNB only. Dual tracer technique was used in 545 of the 689 patients (79%) who received SLNBs. At least one sentinel node was found in 639 of these 689 patients (92.9%), with only one sentinel LN found in 78 patients (12%). Among the 525 cN1 patients in whom 2 or more SLNs were found, 215 (40.9%) had a complete nodal response. Of the remaining 310 patients found to have residual disease on ALND, 39 had a negative SLNB, yielding a false-negative rate of 12.6%, higher than the preset threshold for the trial of 10%.
SENTINA (SENTinel NeoAdjuvant) was a multicenter, prospective cohort study with more than 1000 patients from Austria and Germany conducted to determine the optimal timing of SLNB relative to neoadjuvant chemotherapy. Patients were accrued to one of several arms. Women with clinically node-negative (cN0) disease underwent SLNB before neoadjuvant chemotherapy were enrolled into Arm A. Arm B consisted of patients who were cN0 patients but found to have a positive SLNBs (pN1) and underwent a second SLNB after neoadjuvant chemotherapy. Women who presented with clinically node-positive disease (cN+) received neoadjuvant chemotherapy without preceding SLNB; those cN+ patients who went on to experience clinical pCR (i.e., became ycN0) received postchemotherapy SLNB and ALND (Arm C), whereas those who remained clinically node-positive (ycN+) undergoing ALND alone (Arm D). Compared with Z071, the false-negative rates of SLNB in SENTINA patients after neoadjuvant chemotherapy were higher. In Arm B patients, posttreatment SLNB had a false-negative rate of 51.6%, whereas Arm C patients with pretreatment cN+ disease that became ycN0, the false-negative rates of SLNB after chemotherapy were 24.3% for women who had one sentinel node removed and 18.5% for those who had two nodes removed.
The findings from both ACOSOG Z1071 and SENTINA indicate that, given current practices and patient selection patterns, SLNB alone is not an appropriate alternative to ALND for axillary staging after neoadjuvant chemotherapy in patients with cN1 disease. However, there are adjunct clinical practices that could better identify patients in whom selective axillary surgery after neoadjuvant chemotherapy is more or less likely to be successful and sufficient. A secondary goal of ACOSOG Z1071 is a determination of axillary ultrasound (AUS) accuracy in LABC after completion of neoadjuvant chemotherapy. In a secondary analysis in which AUS images in 611 patients from this trial were reviewed, patients with axillary nodes that appeared suspicious on AUS had more positive nodes and larger nodal metastases compared with patients with sonographically normal appearing nodes. Thus a strategy in which SLNB was only performed on patients with nodes that appeared normal on AUS was modeled and projected to potentially reduce the false-negative rate among cN1 patients from 12.6% to 9.8%, that is, below the previously prescribed threshold of 10%. However, these findings need further clinical validation before AUS is routinely adopted to screen patients at low risk for false-negative SLNB.
Another method of reducing the false-negative rate of selective axillary surgery after neoadjuvant chemotherapy is through targeted dissection of lymph nodes that had previously been found to harbor disease via pretreatment, ultrasound-guided FNA and that were marked with a clip at the time of biopsy. In a prospective study at MD Anderson, previously clipped nodes with pretreatment evidence of metastatic disease were localized with radioactive I-125 seeds and removed at the same time as sentinel nodes localized via traditional intraoperative mapping; ALND was subsequently performed. The combined excision of both sentinel and seed-localized clipped nodes, together known as targeted axillary dissection (TAD), yielded a false-negative rate of only 2%.
Thus both AUS and TAD have the potential to shift the standard of care for LABC away from one in which ALND is mandatory for all patients and toward a more individualized approach in which ALND and its concomitant morbidity are safely avoided in selected patients. As of now, however, a complete level I and II ALND is still recommended for definitive staging and control of axillary disease for patients with LABC treated with neoadjuvant chemotherapy. Furthermore, although some centers continue to perform SLNB before commencing neoadjuvant systemic therapy, we recommend that AUS with selective FNA of suspicious nodes be performed for pretreatment axillary staging in LABC.
Timing of Therapies
There is a biological rationale for the sequence of modalities used to treat LABC. Neoadjuvant chemotherapy is used first in an attempt to achieve early control of distant micrometastases. Even though distant disease may be undetectable with current clinical diagnostic procedures, its presence is suggested by the high percentage of distant failures that occur even when complete local control is achieved with radiotherapy and surgery, as demonstrated by preliminary studies in the prechemotherapy era. In addition, the use of neoadjuvant chemotherapy may decrease primary tumor bulk sufficiently to convert some inoperable patients into candidates for mastectomy or BCS. Tumor responsiveness has also been shown to correlate with overall survival, and tumor responsiveness to neoadjuvant chemotherapy may serve as an in vivo chemosensitivity assay to indicate the potential effectiveness of individual agents.
If the tumor increases in size after an initial course of neoadjuvant chemotherapy, second-line chemotherapy is considered. If there is still no response to chemotherapy, surgical resection is considered if feasible. Otherwise, radiotherapy is used, followed by surgical resection. If the tumor has responded to neoadjuvant chemotherapy, surgery is then used as the second modality. At our institution, pathologic assessment of response to neoadjuvant chemotherapy is used as an indicator of prognosis. In addition, residual cancer burden (RCB), a prognostic index comprising primary tumor (size and cellularity) and nodal disease measures (number and size) derived from the surgical specimen, can lend granularity to the wide range of response that exists between pCR and chemoresistant disease by delineating categories of response—RCB-I (minimal RD), RCB-II (moderate RD), and RCB-III (extensive RD)—that correlate with likelihood of recurrence. Thus pathologic review of the surgical specimen can help guide radiotherapy plans as well ( Box 59.3 ). Radiotherapy is optimally performed at the completion of both surgical resection and chemotherapy, and the rationale for this sequence is further supported by the fact that radiotherapy and doxorubicin are poorly tolerated if given simultaneously. In addition, local control is equally good with early or late radiotherapy, but more distant failures have been observed with postoperative radiotherapy given before adjuvant chemotherapy.
Carcinoma
- •
The chest wall and peripheral lymphatics are treated to 50 Gy in 25 fractions.
- •
The chest wall is treated with either tangential photon-beam or electron-beam fields, depending on patient anatomy.
- •
When the chest wall is treated with tangents, a matched 15- to 20-degree obliqued electron field is used to treat the medial chest wall and internal mammary lymph nodes.
- •
A matched obliqued anterior photon field is used to treat the Rotter space, the level III axilla, and the supraclavicular lymph nodes. For patients with incomplete axillary dissection, a dose supplement is used to treat the level I and II axillary lymph node region. Three-dimensional treatment planning with contouring of structures/targets is used to ensure that targets are adequately covered by prescription dosages.
- •
The chest wall including the mastectomy scar and areas of gross undissected adenopathy receive a boost of 10–16 Gy.
- •
Patients with inflammatory carcinoma are treated on an accelerated, hyperfractionated (twice-daily) schedule unless they achieve a significant pathologic response to chemotherapy.
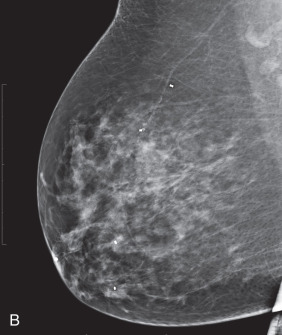
The issue of response versus progression of the primary tumor is assessed by physical examination and repeat imaging, which can include mammography, ultrasonography, and/or MRI ( Fig. 59.4 ). Established criteria of the Union Internationale Contre le Cancer are used to grade tumor regression. Surgical treatment consists of either total mastectomy or segmental mastectomy with level I/II axillary dissection as previously described. Surgical resection is generally performed 3 to 4 weeks after the last chemotherapy treatment to allow time for recovery from the myelosuppressive effects (e.g., granulocyte and platelet nadirs) observed in the 2 weeks after chemotherapy. In the event of prolonged myelosuppression, complete blood cell counts are followed until the granulocyte count is greater than 1500/mm 3 , at which time surgical resection is performed.