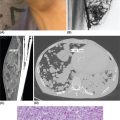
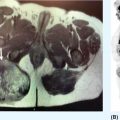
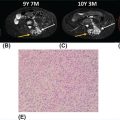
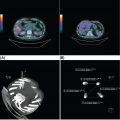
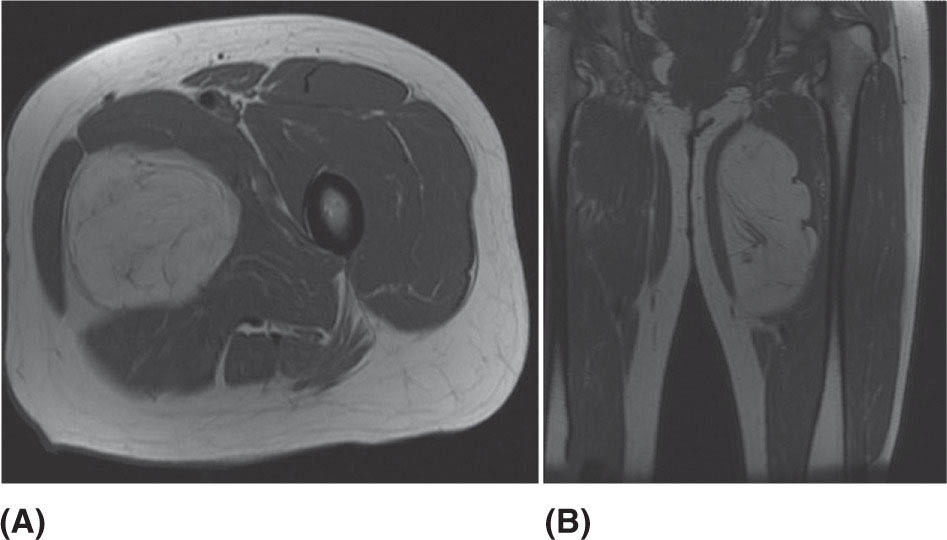
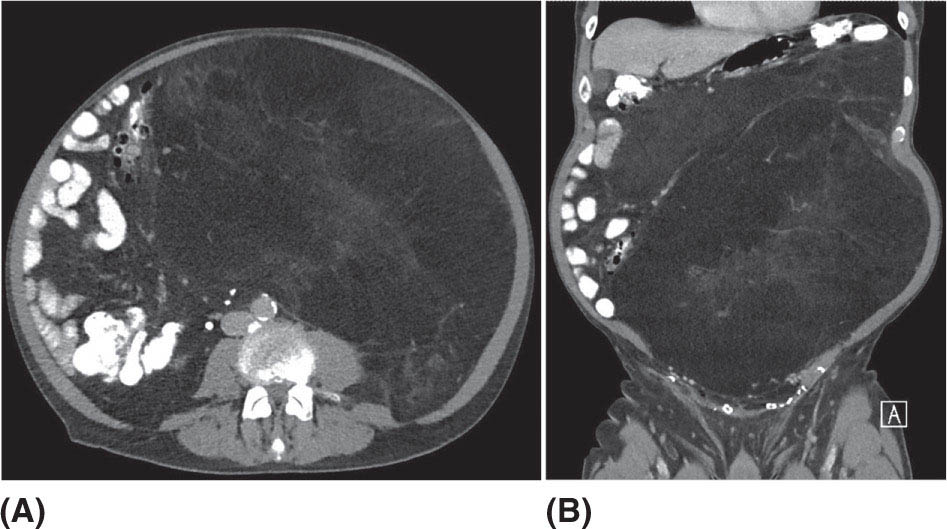
132 Surgical Oncology Soft tissue sarcomas are composed of a heterogeneous group of tumors that demonstrate distinctive clinical behavior based on their inherent tumor biology. As a result, a histology-specific, site-specific approach is recommended. This chapter discusses site-specific surgical principles and highlights histology-specific factors that should assist in guiding the operative approach. The rarity and complexity of soft tissue sarcomas require multidisciplinary evaluation at a sarcoma center in order to determine optimal therapy and maximize outcomes. Preoperative biopsy and confirmation of accurate diagnosis are paramount in most scenarios and allow a histology-specific approach to treatment. Understanding of the patterns of local and/or distant failure should be one component that helps guide the appropriate extent of resection. The optimal extent of resection must be carefully weighed against the associated risks of morbidity and mortality. Surgery plays a significant role in the multimodality treatment of recurrent or metastatic disease in carefully selected patients. Close postoperative follow-up and surveillance are required to identify recurrent disease promptly. sarcoma, surgery, margins, retroperitoneal, extremity, desmoid morbidity, mortality, postoperative follow-up, preoperative biopsy, soft tissue sarcomas, surgery, tumors Aftercare, Biopsy, General Surgery, Morbidity, Mortality, Sarcoma INTRODUCTION Soft tissue sarcomas (STSs) are a rare group of mesenchymal tumors that arise from fat, smooth/skeletal muscle, blood vessels, and connective tissue throughout the body. Gastrointestinal stromal tumors (GISTs) are the most common subtype of sarcoma among a heterogeneous group of over 70 different histologic subtypes.1 There are approximately 13,000 new diagnoses expected in 2018 in the United States, occurring most commonly in the extremities (60%) followed by the retroperitoneum/abdomen (20%), trunk (10%), and head and neck region (10%).2,3 A macroscopically complete resection (R0/R1 resection) is the standard of care for patients with localized STS. There are no data demonstrating a survival benefit for wider pathologic margins. This often requires en bloc resection of the tumor and involved adjacent structures. An R2 resection or resection with gross residual disease should be avoided. Because of the rarity of these tumors, a multidisciplinary evaluation at a high-volume sarcoma center is paramount and has been shown to be associated with improved overall outcomes.4 After initial radiographic evaluation with either contrast-enhanced CT or MRI, a percutaneous core-needle biopsy (12–16 gauge) is often required to guide the consideration of preoperative chemotherapy and radiation therapy, as well as guide the scope of operation based on histologic subtype and grade. The primary site of origin of STS is a significant prognostic factor, with patients with retroperitoneal sarcomas (RPSs) generally having a worse prognosis than those with extremity soft tissue sarcomas (ESTSs). Death in patients with RPS is often secondary to local failure, while death in patients with ESTS is often due to distant failure. This difference in outcome is likely influenced not only by variations in distribution of histologic subtypes, but also by anatomic factors associated with the typically large size of tumor at the time of presentation and frequent multivisceral involvement of RPS. Surgical management of STS should be guided not only by the primary site of origin but also by another key prognostic factor, the histologic subtype. Histologic subtype significantly influences patterns of local and distant failure in addition to survival outcomes.4,5 As a result, instead of a “one-size-fits-all” approach to the surgical management of STS, a histology-specific, site-specific approach is recommended. This chapter discusses site-specific surgical principles and highlights histology-specific factors that should assist in guiding the operative approach. EXTREMITY AND TRUNCAL SARCOMA Extremity and truncal STS often present as a palpable mass and can vary considerably in size at the time of presentation from a small superficial mass to a large, deep compartment thigh mass. After a thorough history and physical examination, radiographic evaluation by MRI (or CT) can be helpful in determining the extent of soft tissue involvement and identify any critical adjacent structures. Imaging alone is very rarely sufficient enough for diagnosis, and percutaneous biopsy is generally recommended. One exception to this is in the case of an atypical lipomatous tumor (ALT)/well-differentiated liposarcoma (WDLPS), which is characterized by a well-encapsulated, homogeneous fat-dense mass, often with thick internal septations (Figure 2.1A and 2.1B). Preoperative biopsy may be deferred in this case with upfront resection of the mass to include a thin rim of normal muscle, fat, or fascia. When biopsy is obtained, a 12- to 16-gauge needle is recommended for a core-needle biopsy, because fine-needle aspiration rarely yields sufficient cellular material for diagnosis and does not maintain enough tissue architecture to distinguish between types of sarcoma, which is critical to optimal treatment planning. 14A core-needle biopsy should be performed using image guidance by an interventional radiologist in order to ensure the solid component of a mass (as opposed to a necrotic or hemorrhagic component) is the target of biopsy when present. While the risk of needle tract seeding is exceedingly rare, the needle tract is typically oriented such that it will be incorporated in either the planned surgical incision or radiation field, though this is not mandatory. An incisional biopsy is rarely indicated; however, when required, the incision should be placed along the long axis of the extremity such that this incision can be incorporated into the incision used for definitive resection. FIGURE 2.1 (A) Axial and (B) coronal images from MRI demonstrating homogeneously T1-hyperintense mass with hypointense internal septations suggestive of an atypical lipomatous tumor. The landmark randomized controlled trial performed by Rosenberg et al. in 1982 significantly changed the treatment paradigm for ESTS.6 The results of this trial shifted the historical standard of care from amputation to a limb-sparing approach with no significant difference in overall survival (OS). Optimal outcomes are achieved with multidisciplinary evaluation with utilization of neoadjuvant/adjuvant chemotherapy and/or radiation therapy for tumors with a high risk of local or distant recurrence. The goal of surgery is a function-preserving, limb-conserving resection with negative microscopic margins (R0 resection). Although the data on margin status and survival are conflicting, negative microscopic margins have been shown to significantly improve local control rates.7–10 Local control and survival may be preserved in the setting of an anticipated positive margin adjacent to a critical underlying structure when combined with preoperative radiation therapy.11 The surgical management of ESTS begins with a critical review of preoperative imaging in order to guide placement of incision in relation to the mass and adjacent critical structures. Understanding of functional anatomy is required in order to optimally balance oncologic resection and postoperative functional status. Resection should include the mass with a 1- to 2-cm rim of adjacent subcutaneous fat, muscle, or fascia, although narrower margins suffice when an anatomic barrier (such as fascia) is removed or radiation therapy is also used. Resection of overlying skin may be required and should be anticipated in order to coordinate potential reconstruction with a skin graft, rotational flap, or free flap. If a mass is superficial to the fascia, the fascia can be resected as the deep margin. When the underlying fascia is involved, the fascia should be resected along with the rim of underlying uninvolved muscle. Tumors abutting critical blood vessels, nerves, bone, and other structures require multidisciplinary evaluation as preoperative radiation and chemotherapy may allow these critical structures to be spared from en bloc resection without compromising oncologic outcome.11,12 When en bloc resection is required, for instance, when a structure is encased by tumor and salvage would require disruption of tumor capsule, vascular reconstruction with an autologous or synthetic graft and nerve reconstruction with an interposition graft can be performed. For periosteal involvement without evidence of bone invasion, the periosteum can be resected as a margin. Prophylactic stabilization of the bone to prevent future fracture can be considered depending on the extent of periosteal stripping as well as the total radiation dose and the degree of circumferential exposure within the radiation field.13–15 All specimens should be oriented with marking sutures for pathologic examination. Titanium clip placement at the margins of the tumor bed can guide planning of postoperative radiation therapy if indicated. Atypical Lipomatous Tumor ALT is the histologic equivalent of WDLPS of the retroperitoneum. ALTs have a homogeneous fat-dense appearance on imaging and may be difficult to distinguish from an intramuscular lipoma. ALTs 15are low-grade tumors; however, they have a significant local recurrence risk of 20% to 30% with a negligible risk of distant recurrence. While a negative microscopic margin remains the goal, the slow-growing nature of these tumors may allow for a focally positive margin over critical structures in order to preserve limb function. A not uncommon scenario is an incidentally discovered ALT following a marginal excision of a presumed lipoma. Baseline imaging can identify the presence and extent of residual disease. When ALT recurs, the disease-free interval can be long and the growth rate can be slow, so further intervention can be delayed for years and patients can be observed, provided no solid tumor tissue is noted within the area of recurrence on yearly MRI studies. Resection for recurrence may be reserved for those with progressing or symptomatic disease. Myxofibrosarcoma Myxofibrosarcomas demonstrate a characteristic growth pattern with a propensity for multiple local recurrences. Commonly arising in the extremities of elderly patients, these tumors have a tendency to infiltrate the surrounding soft tissue with fingerlings often extending beyond the primary appreciable mass of the tumor. When arising intramuscularly, they may be confined by fascial boundaries, but they can frequently demonstrate invasion into adjacent compartments. The microscopic extensions can travel along fascial planes and neurovascular structures and can invade through fascia separating the muscle compartments. Despite local recurrence rates ranging from 18% to 31%, the 5-year OS rates have been reported to be up to 77%.7,16,17 Multiple local recurrences not only limit limb-salvage approaches but often demonstrate higher histologic grade than the primary lesion and have a greater metastatic propensity.18 This highlights the need for aggressive upfront local therapy. Resection should aim for wider margins than other sarcomas, typically 2 to 4 cm around the appreciable mass, and is typically combined with either neoadjuvant or adjuvant radiation and/or chemotherapy. Management of the resulting defect often requires complex closure by a reconstructive surgeon. Closure may be delayed by the use of interim dressings, such as a negative pressure wound therapy dressing, to allow pathologic confirmation of negative margin status as well as margin re-excision in the event of positive margins prior to final wound closure. Myxoid Liposarcoma Myxoid liposarcoma is the second most common type of liposarcoma after WDLPS. In contrast to most ESTS, which predominately metastasize to the lungs, myxoid liposarcomas demonstrate a unique tumor biology with a predilection for extrapulmonary distant metastases.19 Distant metastases occur in approximately one-third of patients, with over half of these patients developing bony involvement, with a propensity for the spine.20 MRI of the spine should be included as part of the staging workup, in addition to CT imaging of the chest, abdomen, and pelvis in these patients. The favorable 5-year local recurrence-free survival (RFS; 97.7%) and metastasis-free survival (89.1%) rates are likely a reflection of their particular sensitivity to chemotherapy and radiation therapy.21 Fibromyxoid Sarcoma Fibromyxoid sarcoma is a low-grade sarcoma that commonly afflicts young male adults, with a propensity for the deep soft tissues of the proximal lower extremities. In contrast to their relatively bland histologic appearance, these tumors demonstrate significant rates of local and distant recurrences, with most recent studies reporting rates of 64% and 45%, respectively.22 Resection should include a 1- to 2-cm rim of normal adjacent tissue when feasible. Local recurrence and distant metastases have been documented as late as 15 and 45 years, respectively, and emphasize the need for prolonged surveillance in these patients. Undifferentiated Pleomorphic Sarcoma Undifferentiated pleomorphic sarcoma (UPS; pleomorphic fibrosarcoma, pleomorphic myxofibrosarcoma) is exclusively an intermediate- or high-grade tumor that was previously categorized under the heading “malignant fibrous histiocytoma.” These aggressive tumors are the most common histologic subtype of ESTS and are typically diagnosed in older adults in their sixth and seventh decades of life. UPS has the worst oncologic outcomes of the histologic subtypes arising in the extremity. While these tumors have been reported to demonstrate comparable 5-year local RFS (81.3%) to other common histologic subtypes, 5-year distant metastasis-free survival (DMFS; 56.8%) and disease-specific survival (DSS; 55.6%) are significantly inferior.23 High rates of unexpected R1 and R2 resections highlight the necessity for multimodality therapy. 16RETROPERITONEAL SARCOMA The retroperitoneum is the primary site in approximately 15% to 20% of patients with STS. The primary site of STS is a known prognostic factor with those arising in the retroperitoneum having a worse prognosis than those arising in the trunk or extremity.24 Tumors of the retroperitoneum present a unique technical challenge owing to their typically large size at presentation and close proximity to critical structures and often require a multivisceral resection. Patients typically present with a new asymptomatic palpable abdominal mass or as an incidental finding during workup of nonspecific abdominal complaints. Initial radiographic evaluation with contrast-enhanced CT can provide details regarding the extent and involvement of adjacent structures, evaluate for metastatic disease, and potentially provide diagnostic information based on histology-specific imaging characteristics. Percutaneous image-guided biopsy is generally recommended in order to confirm diagnosis and histologic subtype, which will guide multidisciplinary evaluation with medical and radiation oncology. A biopsy may not always be warranted, as in the case of preoperative imaging demonstrating a homogeneous fat-dense mass that is suggestive of a WDLPS (Figure 2.2A and 2.2B). In this case, diagnostic information from a biopsy would likely not change the treatment approach. While there is a theoretical risk of needle track seeding with a biopsy, the reported incidence following biopsy is <2%.25 The goal of surgery for RPS is a macroscopically complete (R0/R1) resection of the mass with en bloc resection of directly involved adjacent structures. In contrast to the surgical approach for sarcomas of the extremity where a 2-cm rim of uninvolved surrounding muscle can usually be resected with limited additional morbidity, the close proximity of critical structures in the retroperitoneum requires a balance of oncologic principles and the morbidity associated with resection of these structures. As a result, the appropriate extent of resection and the need to resect adjacent uninvolved structures has been a topic of much debate. Suboptimal 5-year local RFS rates ranging between 42% and 59% despite an R0/R1 resection have prompted the exploration of a more aggressive surgical approach in order to provide improved local control. Some have advocated a compartmental resection approach, which involves a liberal en bloc resection of uninvolved organs in order to obtain a rim of normal tissue surrounding the tumor.26–28 This more aggressive approach has demonstrated significantly improved local recurrence rates; however, no difference in OS has been observed. While more aggressive surgery may improve R0 resection rates, the prognostic significance of converting an R1 to an R0 resection remains unclear. While the extent of resection continues to be debated, there are several key surgical principles recognized for RPS. Technical principles and consensus guidelines for the surgical management of RPS have been described.29–31 A comprehensive knowledge of the retroperitoneum borders and structures is required in order to guide operative planning as well as to respect anatomic distortions due to the typically large size of these tumors. Anatomic considerations such as involvement of end organ vascular supply and organ encasement must be appreciated. Exposure and operative approach are paramount. Multiple incisions can be used based on the size and location of the mass. Choice of incision should be based on surgeon preference, while maximizing exposure in order to allow manipulation of the mass without disruption of the tumor capsule. A midline incision can access the left or right retroperitoneum with mobilization of intra-abdominal structures, though exposure of the subdiaphragmatic regions and the deep aspects of the retroperitoneum can be challenging, particularly in an obese patient. Alternatively, with the patient in a lateral decubitus position, a lower midline incision that is extended obliquely across the upper abdomen toward the tip of the ninth or 10th rib can provide optimal exposure for tumors involving the right or left upper quadrants and diaphragm, though this has the downside of cutting across the upper abdominal musculature. This incision can then be extended into the chest as part of a thoracoabdominal incision, if needed. For tumors located in the lower retroperitoneum or lateral side of the pelvis, either a transverse flank or modified Gibson incision can provide extraperitoneal access for smaller, low-grade tumors along the spine, psoas, pelvic sidewall, or sciatic notch. FIGURE 2.2 (A) Axial and (B) coronal CT images of large homogeneous fat-dense mass with radiographic features consistent with a retroperitoneal WDLPS. WDLPS, well-differentiated liposarcoma. 17TABLE 2.1 Classification System for Rationale for Organ Resection When considering the need for organ resection, a histology-specific approach should be considered as opposed to a “one-size-fits-all” compartmental resection approach. In contrast to defining the “optimal” extent of resection, a recommended “minimal” extent of anticipated resection can be guided based on histology-specific tumor biology and the likelihood of local invasion. Two recent large studies (>600 patients) have reported histology-specific outcomes for patients undergoing surgery for primary RPS, which have broadened the understanding of the distinct tumor biology associated with individual histologic subtypes.4,5 Both of these landmark studies demonstrated that local and distant failures are significantly influenced by histologic subtype. Additionally, histologic subtype can also predict the likelihood of histopathologic organ invasion (HOI), and thus necessitate the need for adjacent organ resection.32 Recently reported data have demonstrated that HOI is present in 26% of resected adjacent organs and is an independent predictor of worse 5-year OS. The likelihood of HOI is influenced by histologic subtype, with patients with dedifferentiated liposarcoma (DDLPS), leiomyosarcoma (LMS), and WDLPS found to have HOI rates of 61%, 56%, and 40%, respectively. Rationale for organ resection may further predict the likelihood of HOI when classified using a six-tier classification system (Table 2.1). HOI has been shown to be present in 65% of organs resected when the surgeon felt the tumor was frankly invading the organ intraoperatively.33 When a tumor is encasing or adherent to an organ, HOI is present in 19% and 26%, respectively. Well-Differentiated Liposarcoma WDLPS is a low-grade malignancy and is the most common subtype of liposarcoma. WDLPS is characterized by a negligible risk of distant failure, but a significant local recurrence risk of approximately 25% at 8 years, which remains constant over time. Surgical management of these tumors and the extent of resection should reflect their less aggressive tumor biology. The lower likelihood of HOI may justify avoiding nephrectomy when possible, as long as the tumor could be removed without violating the tumor capsule. While local failure is the primary cause of disease-specific death, there is typically less of a need for adjacent organ resection, knowing that a local recurrence can often be managed with observation, radiation therapy, or additional surgery. Dedifferentiated Liposarcoma DDLPSs are associated with a more aggressive tumor biology characterized by a significant risk of both local and distant failure. These tumors demonstrate a high rate of HOI that necessitates a more aggressive approach to adjacent organ resection. Multimodality therapy should be considered. The results of the recently completed European Organization for Research and Treatment of Cancer (EORTC) Phase 3 trial (STRASS) will better define the role of neoadjuvant radiation therapy in the local control of RPS.34 Efforts to investigate the utility of systemic chemotherapy in the management of these patients are in development. Review of preoperative imaging is critical in order to appreciate any surrounding well-differentiated homogeneous fat-dense component in addition to the more solid and heterogeneous dedifferentiated component. A common problem noted in sarcoma centers is that patients 18may undergo surgery for a presumed renal cell carcinoma or ovarian cancer, in which a solid mass is removed, and it then turns out to be DDLPS. The surrounding abnormal fat consistent with WDLPS was not originally recognized, and thus was left behind as a large volume of residual disease. Leiomyosarcoma LMS is an aggressive histologic subtype that is thought to originate from a smooth muscle lineage. LMS is characterized by a relatively low risk of local recurrence but a very high risk for distant metastases. An approach to the primary tumor less aggressive than for DDLPS may be justified when the overall outcome in these patients will be ultimately dictated by distant metastases (though an R0/R1 resection, with a margin where possible, is still indicated). These tumors can be technically challenging when arising from the smooth muscle cells of the inferior vena cava or renal vein. The surgical approach for LMS of vascular origin often requires consultation with a vascular surgeon to aid with reconstruction depending on the extent of resection and intraoperative findings. Solitary Fibrous Tumors Solitary fibrous tumors (SFTs) are a rare group of sarcomas that are characterized by a more indolent natural history when arising in the retroperitoneum. While malignant and dedifferentiated variants demonstrate more aggressive behavior, the less aggressive classical variant of SFT predominates in the retroperitoneum. These tumors are typically associated with low rates of local recurrence and distant metastasis.35,36 Schwannoma Schwannomas are benign nerve sheath tumors that can occur sporadically or as a manifestation of neurofibromatosis types 1 and 2. Schwannomas are the most common benign tumor arising in the retroperitoneum. These tumors often present as incidental findings on cross-sectional imaging, and percutaneous biopsy is usually warranted in order to rule out more common malignant retroperitoneal tumors. Schwannomas can present a significant technical challenge when arising from a named nerve or from a sacral nerve root at the level of a foramen. The benefit of resection must be carefully weighed against the potential morbidity associated with resection. A subtotal resection can be considered in order to limit morbidity because of the near-negligible risk of local recurrence or malignant transformation. VISCERAL SARCOMAS Sarcomas arising from intra-abdominal viscera demonstrate a distinct clinical behavior and require a histology-specific treatment approach. The two most common visceral sarcomas are intra-abdominal visceral LMS and GIST. The discovery that most GISTs express the receptor tyrosine kinase KIT (CD117) by Hirota et al. in 1998 led to more accurate distinction between these two subtypes and, more importantly, transformed the management of GISTs with the development of tyrosine kinase inhibitors.37 Gastrointestinal Stromal Tumor GIST is the most common subtype of sarcoma and accounts for 18% of sarcoma diagnoses. The management of GIST is unique compared to other sarcoma subtypes owing to the presence of the gain-of-function c-kit mutation and subsequent efficacy of targeted tyrosine kinase inhibitors in the treatment of these tumors. With c-kit mutations present in approximately 85% of GISTs, imatinib mesylate (Gleevec; Novartis, Basel, Switzerland) is considered the standard of care in the neoadjuvant and adjuvant setting in appropriately selected patients. A macroscopically complete resection is the cornerstone of the management of GIST as it is the only potentially curative therapy. Preoperative workup should include contrast-enhanced CT imaging of the abdomen and pelvis. Routine imaging of the chest for staging is not required because of the rarity of pulmonary metastases. Percutaneous biopsy is typically warranted in order to confirm diagnosis and potentially guide the use of neoadjuvant imatinib therapy. Diagnosis can be confirmed on immunohistochemistry with characteristic expression of markers including KIT (CD117, 85%), CD34 (60%–70%), smooth muscle actin (30%–40%), and S-100 (5%).38 Additionally, DOG1 expression is present in >95% of GISTs, is rarely expressed on other tumors, and can identify KIT-negative GISTs with/without PDGFRA mutations.39 19Surgery should begin with careful exploration of the abdomen in order to identify any undetected metastatic disease. As is true with RPSs, exposure is critical, so that the tumor can be removed with limited manipulation in order to avoid rupture of the tumor capsule. Wide margins are not required for GIST and due to the low incidence of lymph node metastases, lymphadenectomy is not required. While an R0 resection is optimal, similar rates of RFS have been demonstrated after R1 resection. Typically, localized tumors involving the stomach and small bowel can be approached with a wedge or segmental resection. More extensive resections may be required for tumors involving the proximal stomach/gastroesophageal junction, duodenum, and rectum. For these technically challenging scenarios, the use of neoadjuvant imatinib can be considered to facilitate a less extensive resection. Neoadjuvant imatinib also has utility in potentially downstaging a tumor in order to allow a laparoscopic approach. A laparoscopic approach for gastric GISTs of 2 to 5 cm has been shown to be safe without compromising oncologic outcomes and has subsequently been endorsed by the National Comprehensive Cancer Network (NCCN) guidelines. Outcome data for nongastric sites are sparse and it should only be attempted by oncologic surgeons with an advanced laparoscopic skill set. GISTs that are 2 cm and smaller require special mention as these are often discovered incidentally and the majority likely do not require surgical resection. Resection of these small GISTs should be considered in the presence of high-risk endoscopic features such as irregular extraluminal border, heterogeneous echo pattern, presence of cystic spaces, and echogenic foci. In the absence of these findings, currently, the NCCN guidelines recommend endoscopic surveillance every 6 to 12 months. Endoscopic removal is generally discouraged because of increased risk of positive margins, tumor spillage, and perforation. All patients should be evaluated for the need for adjuvant imatinib. Multiple prospective, randomized clinical trials have evaluated the efficacy and duration of adjuvant imatinib.40–42 The need for adjuvant imatinib is based on the estimation of risk of recurrence, which typically takes into account tumor size, mitotic index, and primary tumor location. Multiple risk calculators have been developed, and in general, at least 36 months of adjuvant imatinib is recommended for patients with intermediate or high risk of recurrence. All patients who undergo neoadjuvant imatinib treatment should be continued on adjuvant imatinib for a total of 36 months of combined treatment owing to the inability to accurately assess recurrence risk from the treated surgical specimen. Treatment therapy and dosing of imatinib can be guided by genotype analysis. While the standard dosing of imatinib is 400 mg/day, extrapolating data from patients with recurrent/metastatic GIST, patients with a KIT exon 9 mutation may demonstrate improved results at a higher dose of 800 mg/day.43 Patients with a succinate dehydrogenase (SDH)-deficient GIST or platelet-derived growth factor receptor-alpha (PDFGRA) D842V GIST are insensitive to imatinib treatment and require alternative treatment strategies. Surveillance imaging with CT imaging of the abdomen and pelvis should be obtained every 3 to 6 months for the first 3 to 5 years and then annually thereafter. Routine surveillance imaging of the chest is not indicated because of the rarity of pulmonary metastases. Visceral LMS Visceral LMS is the most common non-GIST intra-abdominal visceral sarcoma. Intra-abdominal LMS is much rarer than LMS arising in the retroperitoneum and extremities, and available data are generally limited to case reports and case series. Data reported on visceral LMS before the year 2000 are likely confounded by the inclusion of GISTs that were often misclassified prior to the discovery of their diagnostic molecular marker profile. Visceral LMS lacks KIT, CD34, and DOG1 molecular markers, which are present in GISTs, and express smooth muscle markers such as desmin and smooth muscle actin. Initial workup of visceral LMS is similar to that of GIST with contrast-enhanced CT imaging and endoscopic or percutaneous biopsy. These tumors do differ from GISTs in their propensity to demonstrate a higher rate of local recurrence, whereas GIST rarely recurs locally. The goal of surgery is an R0 resection, with wide margins recommended in order to avoid an R1 resection. Inferred from LMS of the extremity, R1 resection is associated with worse survival. Lymphadenectomy is not indicated for visceral LMS. The limited data available for visceral LMS suggest they demonstrate similar behavior to their retroperitoneal counterparts. The risk of local and distant failures has been reported to be 21% and 43%, respectively, at 5 years.44 The lung is the most common site of distant metastases. Notably, distant recurrences have been reported in approximately 10% of patients beyond 5 years from original diagnosis, and this highlights the need for continued surveillance with CT imaging of the chest, abdomen, and pelvis beyond the standard 5 years. 20BREAST SARCOMAS Breast sarcomas are a rare group of tumors that can arise from the breast parenchyma or from the skin of the breast. The two most common histologic types are angiosarcoma and phyllodes tumors. These tumors demonstrate distinctive tumor biologic features that guide their specific treatment approach and overall outcomes. Primary Angiosarcoma Primary angiosarcoma arises from the breast parenchyma and is typically seen in young women aged 30 to 50 years. No predisposing risk factors have been identified for primary angiosarcoma. These tumors typically present as a palpable mass and are often diagnosed after a workup including a mammogram, ultrasound, and biopsy. Once diagnosis is confirmed, staging workup should include CT imaging of the chest, abdomen, and pelvis, as well as an MRI study of the breast. Surgery is the only potentially curative treatment for primary angiosarcomas. Unlike conventional breast cancer that may potentially be amenable to a breast-conserving surgical approach when combined with radiation therapy, the sensitivity of breast angiosarcomas to radiation is unknown (although there is no biologic reason that their sensitivity to radiation would be different from that for sarcomas occurring in other anatomic sites). Therefore, simple mastectomy is the preferred approach. On rare occasions when breast conservation may be an option (small tumor in a woman with breast of an appropriate size that lumpectomy is feasible), surgery short of a mastectomy may be reasonable to consider. However, the utility of radiation therapy, owing to the rarity of reported cases, is uncertain. En bloc resection of skin or pectoralis major may be required in order to obtain an R0 resection if in close proximity. Axillary lymph node dissection is not routinely performed because of the low incidence of reported nodal metastases. While angiosarcomas are often responsive to chemotherapy, the role of chemotherapy in the adjuvant setting is unclear as no definitive benefit has been demonstrated, but could be considered in patients at high risk for recurrence and in the neoadjuvant setting to facilitate breast-sparing surgery. Surveillance usually consists of breast/chest wall MRI and CT of the chest, abdomen, and pelvis. Secondary Angiosarcoma Secondary angiosarcomas develop in the setting of prior radiation therapy or chronic lymphedema (Stewart–Treves syndrome). As a result, these patients tend to be older than primary angiosarcoma patients. Radiation-associated breast angiosarcoma often occurs in patients who have undergone breast-conserving surgery followed by radiation for breast carcinoma. This tumor type is distinct from primary angiosarcoma as it arises from the dermis and subcutis in the area of the radiation field. The surgical approach for radiation-associated breast angiosarcoma reflects its inherent tumor biology and is much more extensive than that for primary angiosarcoma. A radical resection, which involves a mastectomy along with resection of all the skin in the radiation field, is required because of the multifocality of these tumors and the fact that the entire area in the radiation field is at risk for disease. This involves removing all the skin extending from the clavicle superiorly, rectus fascia inferiorly, sternal border medially, and latissimus dorsi laterally. Resection of the pectoralis major muscle may be required if it is directly involved, in order to ensure an R0 resection. Collaboration with a reconstructive team is required to facilitate wound closure with skin grafting and/or rotational flap. This radical approach has been shown to reduce recurrence rates and improve DSS when compared to less extensive resection involving only wide local excision or mastectomy with only partial skin resection.45 While local recurrences can be appreciated by physical examination, because of a significant risk of distant recurrence, surveillance imaging should include CT imaging of the chest and abdomen. Phyllodes Tumor Phyllodes tumors are rare fibroepithelial tumors of the breast that can display varying levels of aggressive behavior. While the majority of these tumors are classified as benign, they can also be categorized as borderline and malignant, depending on the degree of stromal cellular atypia, mitotic activity, presence of infiltrative tumor margins, and presence of stromal overgrowth. Phyllodes tumors often present as a large palpable mass in women in their fifth and sixth decades of life. Core biopsy is typically diagnostic, demonstrating a classic leaf-like architecture on pathologic review. The surgical approach involves resection with a margin of at least 1 cm. This may be accomplished with a lumpectomy, depending on the size of the tumor; otherwise, a simple mastectomy is required. Axillary lymph node dissection is not required because of the low incidence of regional lymph node involvement. While not indicated for benign phyllodes tumors, adjuvant radiation may be considered 21in patients with borderline or malignant tumors in order to reduce local recurrence rates.46 The presence of stromal overgrowth is associated with a high risk of distant recurrence and results in consideration for neoadjuvant/adjuvant chemotherapy. Physical examination is adequate for surveillance of a local recurrence and should be combined with CT imaging of the chest, abdomen, and pelvis for borderline and malignant tumors. CUTANEOUS SARCOMAS Sarcomas originating from the dermis and subcutis are a heterogeneous group of tumors that display a wide range of clinical behavior. These tumors may present as a pigmented lesion or subcutaneous nodule that may be difficult to initially distinguish from more common benign and malignant lesions. Dermatofibrosarcoma Protuberans Dermatofibrosarcoma protuberans (DFSP) is a rare cutaneous sarcoma that can demonstrate aggressive local behavior. These tumors often present as a slow-growing mass that may manifest as a pigmented skin papule. They commonly arise in the trunk and proximal extremities and often infiltrate the surrounding soft tissue beyond the appreciable mass. Core biopsy can guide the extent of resection; however, often, DFSP may be an incidental finding after initial marginal resection for an unknown superficial mass. Resection involves wide margins of at least 2 to 4 cm carried down to the level of the underlying fascia. Reconstruction with a skin graft or local flap may be required. Intraoperative margin assessment is challenging as the surrounding fat is not amenable to frozen section analysis. Mohs micrographic surgery is generally not recommended because of lack of long-term results, though it may be considered for tumors in sensitive locations such as the face. Surveillance should include physical examination for detection of a local recurrence. In approximately 5% to 10% of patients, a more aggressive fibrosarcomatous variant will be present. This histologic subtype is associated with a higher rate of local recurrence and propensity for distant metastases. While the surgical approach is similar to that of the classic variant, the behavior of this variant justifies consideration of (neo)adjuvant radiation. Furthermore, surveillance in this subset of patients with the fibrosarcomatous variant may include local imaging with either a chest x-ray or CT imaging of the chest, abdomen, and pelvis. Atypical Fibroxanthoma Atypical fibroxanthoma is a cutaneous sarcoma that often arises in sun-exposed skin of the head and neck of older patients. These tumors typically present as a solitary red papule or nodule that may ulcerate and occasionally bleed. Atypical fibroxanthomas demonstrate fairly benign behavior and require resection of the entire tumor with negative margins. Pleomorphic Dermal Sarcoma Pleomorphic dermal sarcomas are a group of more aggressive cutaneous sarcomas with a greater propensity for both local and distant recurrence. Pleomorphic dermal sarcomas can be difficult to distinguish from atypical fibroxanthomas; however, the former demonstrates invasion into the subcutaneous fat and may demonstrate a higher mitotic count in addition to lymphovascular and perineural invasion. As a result, resection requires wider margins of at least 2 cm. Adjuvant radiation therapy may be considered. Surveillance should include physical examination of the primary site and CT imaging of the chest. DESMOID TUMOR Desmoid tumors are a rare group of tumors that account for <3% of all sarcomas. Also known as desmoid fibromatosis or aggressive fibromatosis, these tumors are histologically benign and lack the ability to metastasize. Desmoid tumors can demonstrate variable clinical behavior and have the ability to be locally aggressive with high local recurrence rates after wide local excision. Desmoid tumors also demonstrate a propensity to occur during pregnancy. Core-needle biopsy can confirm diagnosis and aid in directing management strategy. While the majority of desmoid tumors occur sporadically, approximately 10% develop in the setting of a germline mutation in the adenomatous polyposis coli (APC) gene that is associated with familial adenomatous polyposis (FAP). Desmoids have been reported to develop in 10% to 20% of patients 22with FAP, with the majority arising within the abdomen and the abdominal wall. Notably, desmoids in patients with FAP also demonstrate a propensity to arise at incision sites from previous surgery. Intra-abdominal desmoids present a technical challenge as they often involve the bowel mesentery and mesenteric vessels, resulting in significant morbidity. The management of desmoid tumors has evolved with the pendulum swinging away from an aggressive surgical approach to a much more conservative, watchful waiting approach. While some tumors may be locally aggressive, others may demonstrate more indolent behavior and even spontaneously stabilize or regress (approximately 20% spontaneous regression rate). An initial period of observation is recommended for asymptomatic tumors, even when initially resectable. In the setting of progressive or symptomatic disease, multiple options are available, including cytotoxic chemotherapy (doxorubicin ± dacarbazine, liposomal doxorubicin, vinorelbine + methotrexate), tyrosine kinase inhibitors (imatinib, sorafenib), nonsteroidal anti-inflammatory drugs (indomethacin, sulindac), and antihormonal agents (tamoxifen). Surgery is typically reserved for refractory tumors, especially for mesenteric desmoids, where surgery can be associated with significant morbidity. In the case of pregnancy-associated abdominal wall desmoids, surgery can be considered earlier following a period of observation because of favorable outcomes following resection in this cohort of patients. RECURRENT SARCOMA While surgery is the mainstay treatment for most STS, despite a successful resection with negative margins, recurrence rates remain significant. Systemic therapies may be the first-line treatment in patients with recurrent disease, particularly those with high-grade tumors. This provides the opportunity to gain control of the disease and allow time to understand the tumor biology and disease trajectory. Surgery in the setting of recurrent disease may be considered in a select group of patients. It is vitally important that these complex patients undergo multidisciplinary evaluation at a sarcoma center in order to optimize outcomes.4,28,29,47 Site- and histology-specific factors should guide the surgical management in these patients. Recurrent RPS The most common cause of death in patients with RPSs is from a local recurrence. The potential survival benefit of surgery must be carefully weighed against the potential morbidity associated with these challenging cases. Resection of a local recurrence or distant metastasis has been shown to significantly improve 5-year OS, compared to those patients who do not undergo resection.48 Longer time to recurrence is a significant predictor of improved OS. The surgical management of patients with localized recurrent disease mirrors that of primary disease. The goal of surgery is a macroscopically complete resection with en bloc resection of involved adjacent organs. Surgery in patients with a multifocal recurrence should be considered for palliative purposes weighed against the poor overall outcomes in these patients. The true survival benefit after resection of distant metastases is unclear and should be addressed on a case-by-case basis. The lungs are a common site of metastases and can be considered for resection in the absence of extrathoracic disease, when the primary tumor is treatable/has been treated, and a complete resection can be achieved.49–51 Surgery is not recommended in patients with a concurrent local recurrence and distant metastasis. Metastatic GIST Metastatic disease is present in up to 50% of patients at the time of presentation with GIST.42 Tyrosine kinase inhibitor therapy is the first-line therapy in patients with metastatic disease. Despite the effectiveness of tyrosine kinase inhibitors in controlling the disease, a complete response is very rare. Additionally, the response to tyrosine kinase inhibitor therapy is not indefinite, with the median time to progression on imatinib being 24 months,52 on sunitinib malate being 6.8 months53 (Sutent; Pfizer, New York, NY), and on regorafenib being 4.8 months54 (Stivarga; Bayer, Leverkusen, Germany). As a result, surgery can play a key role in carefully selected patients, with the aim of prolonging time to progression by removing disease before secondary resistance develops and eliminating resistant clones. The goal of surgery in patients with metastatic GIST is a macroscopically complete resection and should include en bloc resection of involved adjacent organs. Attempts to conduct a randomized clinical trial to study the outcomes of cytoreductive surgery in patients with metastatic GIST have been unsuccessful because of poor patient accrual. Multiple retrospective studies have demonstrated that cytoreductive surgery may result in prolonged progression-free survival in patients with responsive or 23stable disease on tyrosine kinase inhibitor therapy at the time of surgery (when compared to surgery in those with progressive disease).55–59 The morbidity associated with these complex operations must be examined, and prediction of significant postoperative morbidity can be aided by a recently published surgical complexity scoring system.60 A planned R2 resection may be justified for symptom palliation; however, resection of only tyrosine kinase inhibitor–resistant lesions while leaving lesions that are responsive to treatment likely provides no benefit. SUMMARY STSs are composed of a heterogeneous group of tumors that demonstrate distinctive clinical behavior based on their inherent tumor biology. The rarity and complexity of these tumors require multidisciplinary evaluation at a sarcoma center in order to determine optimal therapy and maximize outcomes. Preoperative biopsy and confirmation of accurate diagnosis are paramount in most scenarios and allow a histology-specific approach to treatment. Understanding of the patterns of local and/or distant failure should be one component that helps guide the appropriate extent of resection. Additionally, anatomic considerations and likelihood of adjacent organ involvement should be considered. The optimal extent of resection must be carefully weighed against the associated risks of morbidity and mortality. Close postoperative follow-up and surveillance are required to identify recurrent disease promptly. Surgery can play a significant role in the multimodality treatment of recurrent or metastatic disease in carefully selected patients.
Category
Criteria
1
Frank organ invasion/tumor origin
2
Tumor involving vascular supply
3
Tumor encasement of organ
4
Tumor adherent to organ
5
Tumor adjacent to organ/required for macroscopic complete resection
6
Other (iatrogenic injury requiring resection, incidental resection for another reason)
Surgical Oncology
Mark Fairweather and Chandrajit P. Raut