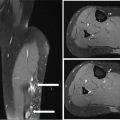
27721 Malignant Peripheral Nerve Sheath Tumor Malignant peripheral nerve sheath tumors (MPNSTs) are soft tissue sarcomas that arise from peripheral nerve sheaths. MPNSTs are aggressive tumors and associated with high frequency of local recurrence and metastatic disease. About half of all MPNSTs arise in patients with neurofibromatosis type 1 (NF1), but tumors also develop in individuals without inherited NF1 and arise with greater incidence in areas following irradiation. Preoperative or postoperative radiation therapy has a role in local control, especially for large tumors. However, high doses of radiation are required for the treatment of MPNSTs, which can be challenging, depending on the location of the primary tumor. The recent clinical and imaging characterization of atypical neurofibromas may allow for earlier diagnosis of lesions at risk of transforming to MPNST and for treatment and prevention strategies. These and other anticipated advances, including the identification of biomarkers of MPNST transformation, will hopefully result in effective prevention and treatment strategies for this devastating tumor. This chapter reviews clinical presentation, driving molecular aberrations, common anatomic location, and general therapeutic approach for MPNSTs. Malignant peripheral nerve sheath tumor (MPNST), neurofibromatosis type 1, atypical neurofibroma, targeted therapies for MPNST, genetic tumor predisposition, genomics, epigenetics biomarkers, clinical evaluation, malignant peripheral nerve sheath tumors, metastatic disease, neurofibromatosis type 1, radiation therapy Biomarkers, Evaluation Study, Neoplasm Metastasis, Neurofibrosarcoma, Radiotherapy INTRODUCTION Malignant peripheral nerve sheath tumors (MPNSTs) are soft tissue sarcomas (STSs) that arise from peripheral nerve sheaths. MPNSTs are aggressive tumors and associated with high frequency of local recurrence and metastatic disease. About half of all MPNSTs arise in patients with neurofibromatosis type 1 (NF1), but tumors also develop in individuals without inherited NF1 and arise with greater incidence in areas following irradiation. To date, complete surgical resection with wide negative margins remains the cornerstone of successful therapy and is required for cure. The objective response rate of MPNSTs to chemotherapy is low and the impact of chemotherapy in the adjuvant or neoadjuvant setting on survival has not been defined. Preoperative or postoperative radiation therapy (RT) has a role in local control, especially for large tumors. However, high doses of radiation are required for the treatment of MPNSTs, which can be challenging, depending on the location of the primary tumor. Several histology-specific Phase II trials with agents targeting pathways implied in the pathogenesis and progression of MPNSTs have been conducted, but have not resulted in clinical activity to date. New insights into the molecular pathogenesis of MPNSTs as well as the availability of disease-specific preclinical models give hope for the development of more effective combination therapies for unresectable or metastatic MPNSTs. In addition, recent insights into the clinical development of NF1-associated MPNSTs may allow for earlier diagnosis of MPNST/MPNST precursor lesions and for the development of MPNST prevention strategies. Most MPNSTs in NF1 develop in preexisting plexiform neurofibromas, which are histologically benign peripheral nerve sheath tumors. In addition to biallelic loss of NF1 in neurofibromas and plexiform neurofibromas, atypical neurofibromas are characterized by heterozygous loss of CDKN2A/B, which appears to be the first step toward malignant transformation. The recent clinical and imaging characterization of atypical neurofibromas may allow for earlier diagnosis of lesions at risk of transforming to MPNST, and for treatment and prevention strategies. As most atypical neurofibromas appear well defined and encapsulated on clinical evaluation and MRI, these lesions are more amenable to surgical removal compared to fully established high-grade MPNSTs. These and other anticipated advances, including the identification of biomarkers of MPNST transformation, will hopefully result in effective prevention and treatment strategies for this devastating tumor. ESTIMATED INCIDENCE MPNSTs account for 4% of all STSs.1 Approximately 50% of all MPNSTs arise in patients with NF1, and the lifetime risk of MPNST for patients with NF1 ranges from 8% to 15.8%,2,3 making MPNST the leading cause of death in patients with NF1.3 Approximately 40% of MPNSTs are sporadic and 10% occur in patients with a history of radiation exposure. The age of diagnosis in NF1-associated MPNST is younger (typically 20–50 years old) with 10% to 20% of cases reported in children (aged 1–19 years), compared to sporadic MPNST, which typically occur in late adulthood.4 Analyses from the Surveillance, Epidemiology, and End Results (SEER) Program indicate a slightly higher incidence of MPNST in males (55.11%) than in females (44.89%), and among all age groups there is an elevated incidence in African Americans compared to Caucasians and other ethnicities.5 CLINICAL PRESENTATION MPNST typically presents as an enlarging mass, which is frequently associated with pain. Neurologic deficits or paresthesia can be associated with the involved nerves. Particular consideration should be 278paid to patients with NF1 and plexiform neurofibroma. These histologically benign tumors are frequently associated with pain. However, a change in pain quality and intensity should be considered as a sign of malignant transformation and trigger further evaluation. In addition, the development of pain or any mass that arises within a prior radiation field should result in consideration for MPNSTs. As complete surgical resection is the only curative therapy for MPNSTs and these tumors can develop rapidly in preexisting plexiform neurofibromas, clinical and imaging evaluation have to be performed in a timely fashion to maximize outcome. A number of prognostic factors have been identified including large tumor size (>5 cm), truncal location, and incomplete surgical resection (reviewed by Farid et al.).6 In several studies, NF1 status has also been associated with worse outcome compared to sporadic MPNST, but not consistently, and a more recent meta-analysis described that this effect is not present in studies after 2000, possibly owing to better surveillance and earlier diagnosis of MPNST in NF1 patients.7 Diagnostic Approach A pretreatment biopsy followed by experienced pathological evaluation is necessary for accurate diagnosis, grading, and management of MPNSTs. This can be done by core-needle or open incisional technique, and ideally the biopsy site should be coordinated with the surgeon who may undertake definitive surgery. Because of the potential for sampling error, especially in MPNSTs developing in a preexisting plexiform neurofibroma, several core-needle biopsies may have to be obtained to avoid false negative results. MRI and fluorodeoxyglucose (FDG)-PET have utility in identifying the site for the biopsy. A recent consensus meeting determined that for lesions that, based on clinical and imaging evaluations, are concerning for atypical neurofibromas but clearly not high-grade MPNST, a biopsy may not be necessary to establish the diagnosis prior to surgical resection of the lesion.8 This includes lesions with distinct nodular appearance, which do not show a recent change in clinical symptoms, rapid growth, or high FDG-PET avidity.8,9 Given that the surgical approaches for high-grade MPNST compared to low-grade MPNST and atypical neurofibromas are different, a biopsy is recommended prior to surgery in all cases in which there is uncertainty of the diagnosis. Pathology of Malignant Peripheral Nerve Sheath Tumors A sarcoma can be diagnosed as MPNST when it has nerve elements or when it develops in a patient with a clinical diagnosis of NF1. Most NF1-associated MPNSTs are high-grade, which is characterized by high cellularity, high mitotic rate—at least 10 mitotic features/10 high power fields (HPFs)—nuclear atypia, and necrosis. In contrast to neurofibromas, most high-grade MPNST have lost S100 and CD34 expression by immunohistochemistry. In addition, loss of trimethylated histone H3 at lysine residue 27 (H3K27me3) has been described as a marker of MPNST with a frequency between 30% to 90%.10 This marker may not be specific for MPNST because it has also been described in other tumors, in particular synovial sarcoma.11 With the clinical and genomic characterization of atypical neurofibromas as precursor lesions to MPNST, a recent pathology consensus review focused on characterizing and providing diagnostic criteria for neurofibromas, neurofibromas with atypia, and low-grade and high-grade MPNST.11 The consensus review proposed the term atypical neurofibromatous neoplasm of uncertain biologic potential (ANNUBP) for lesions concerning for a potentially higher risk for malignant transformation. The term ANNUBP is reserved for lesions meeting at least two of the following four criteria: cytologic atypia, loss of neurofibroma architecture, hypercellularity, and a mitotic index of ≥1/50 HPFs and <3/10 HPFs. The ANNUBP is different from low-grade MPNST, which are characterized by the absence of necrosis but have a mitotic index between 3 to 9/10 HPFs. The review also commented that the distinction between ANNUBP and low-grade MPNST can be difficult and gradual and that the clinical behavior of low-grade MPNST is similar to that of an ANNUBP in that these lesions are at low risk for local recurrence and essentially no risk for metastasis. Radiographic Features and Recommended Imaging In patients with sporadic MPNST, the recommended imaging and diagnostic approach is similar to that for other STSs. Imaging should include detailed imaging of the primary tumor with CT and MRI and metastatic workup including chest CT and FDG-PET scan as appropriate. In patients with NF1, several subgroups have been identified as having a greater risk for MPNST and may benefit from more frequent imaging with the goals of identifying MPNST or MPNST precursors early. Patients with NF1 microdeletion,12,13 high plexiform neurofibroma burden and/or known atypical neurofibroma(s) are at greater risk for developing MPNST.8,9 The role of whole-body MRI in these patients is being studied in the research setting, including the National Cancer Institute’s NF1 279natural history study (NCT00924196). The advantage of whole-body MRI lies in the ability to comprehensively assess growth patterns without exposure to radiation and contrast agents.14 Whole-body MRI also allows monitoring the entire disease burden and several areas of potential concern for malignant transformation. However, while whole-body MRI is feasible, and short tau (T1) inversion recovery (STIR) sequence has been recommended as a core sequence, whole-body MRI is not widely available. In addition, the reproducibility of whole-body MRI and the benefit from prospective longitudinal whole-body MRI is not established yet.15 Imaging evaluation for NF1 MPNST should include MRI with and without contrast and diffusion-weighted imaging to delineate the disease. Imaging should include the surrounding plexiform neurofibroma in its entirety. CT chest/abdomen/pelvis should be performed to evaluate for metastatic disease sites including lungs, liver, and adrenals. FDG-PET imaging has been evaluated extensively for its utility in identifying MPNSTs. Multiple studies have evaluated the qualitative and quantitative (maximal standard uptake value [SUVmax]) assessment of FDG-PET to distinguish neurofibroma from MPNST and have overwhelmingly shown that high-grade MPNST has higher FDG-avidity compared to neurofibromas and thus diagnostic utility.16 Plexiform neurofibromas are rarely FDG-avid, while most high-grade MPNST show increased FDG-uptake with SUV cutoffs of more than 3.517 and 4.018 being proposed to distinguish between plexiform neurofibroma and MPNST. Fewer studies have reported on the FDG uptake in atypical neurofibromas and low-grade MPNST.4,9,17,18 The SUVmax for these lesions can overlap with those seen in high-grade MPNST and plexiform neurofibromas, and more studies will be required to identify if SUVmax for high-grade MPNST and atypical neurofibromas/low-grade MPNST are sufficiently different to be of diagnostic utility. Detailed MRI evaluations have identified size, heterogeneous contrast enhancement, perilesional edema, and perilesional enhancement as predictors for MPNST versus benign nerve sheath tumors. In addition, evaluation of the apparent diffusion coefficient (ADC) has value in predicting for MPNST19 in that lesions with ADC values ≤1.0 × 10−3/s were at 150 times greater risk being an MPNST compared to lesions with ADC values >1.0 ×10−3/s.20 Prospective studies comparing FDG-PET and MRI with diffusion weighted imaging and ADC values may allow further definition for the role of these imaging modalities. MOLECULAR ABERRATIONS DRIVING MPNST Given the high incidence of MPNST within the NF1 population, there have been significant efforts to describe the genetic and molecular mechanisms underlying the malignant transformation of plexiform neurofibroma to MPNST. These efforts have intensified over the past two decades for two reasons. First, clinical observations on the malignant transformation of plexiform neurofibromas defined atypical neurofibromas as a precursor lesion to MPNST. Second, next-generation sequencing methods allowed the complete characterization of the somatic genome underlying these lesions. Genetically, the clinical observations have been matched with mutational profiling studies that have demonstrated sequential and gradual loss of tumor suppressor genes. This work has led to a genetic model, whereby the primary driver of tumorigenesis in plexiform neurofibromas is biallelic loss of NF1 function21 followed by chromosome rearrangement and the loss of CDKN2A (the gene that encodes INK4A/p16) in atypical neurofibromas.21,22 Finally, mutations in EED or SUZ12, components of polycomb repressive complex 2 (PRC2), lead to loss of H3K27me3 and subsequent dysregulation of transcriptional repression.23,24 Somatic alteration of other genes such as RAS25, TP5323,24, and BRAF26 have been reported at varying frequencies.27 While there are no recurrent chromosomal translocations observed in MPNST, cytogenetic and hybridization array studies have demonstrated significant aneuploidy characterizes MPNST tumors. These studies identify recurrent losses of chromosomes 1p, 9p, 11, 12p, 14q, 18, 22q and gains of chromosome 7, 8q, and 15q.28 These findings highlight the potential that MPNSTs may contain inherently unstable genomes, a hypothesis that may have important implications in the age of immunotherapy. An interesting analysis summarizing a relatively large cohort of TCGA data reported a median of 2.5 mutations per megabase in 134 analyzed cases, with less than 10% of MPNST harboring more than 20 mutations per megabase.29 This relatively low mutational burden may reflect the finding of low or absent expression of PDL1 and PD1 within these tumors30 and a disappointing response to checkpoint inhibitors in the handful of reported MPNST treated with these agents.31 Despite these findings, there may be some subsets of patients that will respond to these novel agents, and a Phase II trial of a PD1 inhibitor is currently underway in patients with metastatic or locally unresectable MPNST (NCT02691026). The discovery of alteration of PRC2 members in MPNST has enlightened the understanding of the transcriptomic and epigenetic landscapes of these tumors. The PRC2 complex plays a critical cellular role in maintaining condensed chromatin, mainly by catalyzing the histone modification of H3K27me3 280in specific genomic locations that are transcriptionally silent. PRC2 is involved in various biologic processes, including differentiation, maintaining cell identity and proliferation, and stem-cell plasticity.32 There are three major protein subunits in the complex—SUZ12, EED, and the catalytic EZH2—however, only mutation of SUZ12 and EED have been observed in MPNSTs. Interestingly, those patients with a germline microdeletion of the NF1 locus have a particularly increased risk of MPNST due to concomitant loss of SUZ12, which sits adjacent to the NF1 gene on chromosome 17.24 The observation of IHC correlation between PRC2 mutation and loss of H3K27me3 staining is currently being incorporated into the standardization of histopathologic evaluation of MPNST.10,33 While the transcriptomic consequences of the loss of PRC2 are currently being defined, downstream affected genes include cell specific transcription factors, imprinted genes such as IGF2, and genes associated with development and morphogenesis. All of these disrupted genes may be future therapeutic targets in these tumors.23 The overall disappointing response of MPNST to conventional chemotherapy regimens led to a concentrated effort by the field to identify molecular targets that might represent therapeutic vulnerabilities in MPNST. Given the predominance of MPNST in the NF1 population as well as the presence of somatic mutations of NF1 in the sporadic tumors, targeting of the Ras/Raf/MAPK pathway has been extensively explored both in the preclinical models as well as in the clinic. Tantalizingly, single-agent use of the MEK inhibitor selumetinib was found to be active in plexiform neurofibromas,34 providing further rationale for MEK inhibition to play a central role in the development of combination strategies.35 The PI3K/MTOR/AKT signaling pathway is also hyperactive in NF1 deficient cells36 and multiple in vivo studies have demonstrated the potential utility of targeting this pathway in MPNST.37,38 These preclinical efforts led to the development of a clinical trial studying the combination of selumetinib with the mTOR inhibitor sirolimus for patients with unresectable or metastatic MPNST (SARC031), which recently began enrollment (ClinicalTrials.gov Identifier: NCT03433183). Another potential therapeutic target, further downstream of activated MAPK is Aurora Kinase A (AURKA), which was found to be upregulated in MPNSTs and demonstrated activity both in vitro and in vivo.39 Here, again, the need to develop combination strategies will be important as single-agent targeting of AURKA stabilized tumor volume and provided some improvement in PFS but still resulted in disease progression in the preclinical models. Canonical WNT and beta-catenin signaling are known to be a critical factors in both Schwann cell development and MPNST tumorigenesis40,41 and are another attractive molecular target for therapeutic development. The tumor microenvironment is an additional target for peripheral nerve sheath tumors. Both plexiform neurofibromas and MPNST contain macrophages, which may contribute to tumor progression. Preclinical studies in MPNST models with an agent targeting CSF1 and KIT (PLX3397) in combination with mTOR inhibition resulted in development of a clinical trial (ClinicalTrials.gov identifier: NCT02584647).42 Finally, given the transcriptional dysregulation in MPNST tumors due to activated MAPK signaling and the loss of PRC2, targeting of active enhancers with bromodomain inhibitors, acting on BRD4,43 have emerged as a unique vulnerability of MPNST. Anticipating the need for combination strategies has led to strategies co-targeting MEK or MTOR with BRD444 as well as the use of proteolysis-targeting chimeras (PROTACs) that simultaneously target bromodomain activity, and bromodomain and extraterminal domain (BET) protein abundance.45 Common Anatomic Location and General Therapeutic Approach for MPNST MPNST can arise along any peripheral nerve. The most common locations of primary MPNST are trunk, extremities, and less frequently head and neck. NF1-associated MPNST are more frequently deep seated/truncal in comparison to sporadic MPNST, which is critical, as complete surgical resection in these locations is more difficult to accomplish.4,46 In addition, at diagnosis NF1-associated MPNST are more frequently locally advanced compared to sporadic MPNST.4,46 While at diagnosis, less than 10% of MPNST are metastatic,46 MPNST has a high propensity to metastasize with the most frequent locations of metastasis in decreasing order being pulmonary, bone, liver, abdominal cavity, adrenal gland, diaphragm, mediastinal, brain, ovary, kidney, and retroperitoneum.4,47,48 Complete surgical resection is the only curative therapy in patients with high-grade MPNST.49,50 The surgical goal is wide local resection, which can be difficult and associated with significant morbidity. Amputation, may be required in cases in which negative margins cannot be achieved otherwise, in particular in MPNST arising from major nerves.51 The role of RT has not been studied in prospective trials for MPNST to date. However, a prospective randomized trial of radiation for STSs demonstrated a delay in progression-free but not overall survival in patients receiving radiation.52 Several retrospective studies have described improvement in local control of MPNST with radiation,53 and there is consensus to administer radiation, if feasible, to 281high-grade MPNST, especially if the tumor is larger than 5 cm in diameter.8 Similar to the role of radiation for other STSs, preoperative radiation should be considered, as it has the advantage of a smaller radiation field compared to postoperative radiation.54 The role of proton beam therapy is being studied in STSs, but no studies in MPNST have been reported to our knowledge.55 The role of chemotherapy in MPNST has been prospectively evaluated in very few trials and the impact of chemotherapy on survival in patients with MPNST is not known.1,56 Several retrospective studies have described responses to standard chemotherapy agents, such as doxorubicin and ifosfamide used to treat other STSs.1,4,57 Notably, a retrospective analysis of 12 European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Trials analyzed the outcomes of 175 patients with MPNST out of 2,675 eligible STSs. The outcomes were similar for MPNST and other STSs with a response rate, median progression-free survival, and overall survival of 21% versus 22%, 17 versus 16 weeks, and 48 versus 51 weeks, respectively. Compared to single-agent doxorubicin, the combination of doxorubicin and ifosfamide achieved the best response.58 This study did not compare response for NF1 associated versus sporadic MPNST. However, worse chemotherapy response in NF1 associated MPNST compared to sporadic MPNST has been described in several prior studies and was also associated with worse survival in NF1 patients.49,59 A recent prospective study (SARC006, ClinicalTrials.gov Identifier: NCT00304083) evaluated the role of standard STS chemotherapy in high-grade unresectable MPNST. Patients were stratified by sporadic versus NF1-associated MPNST, with the primary endpoint of determining objective response (complete and partial response) using WHO criteria after two cycles of doxorubicin and ifosfamide followed by two cycles of ifosfamide and etoposide. Etoposide was included in this trial because it is active in pediatric STSs and because topoisomerase IIα, the drug’s target, has been reported as the most overexpressed gene in MPNST compared to benign neurofibromas.60 No complete responses were observed, and the partial response rate was lower in NF1-associated MPNST (17.2%) compared to sporadic MPNST (33%).1 However, this trial did not complete enrollment and was not powered to detect a difference in response rates between the two strata. Importantly, in contrast to trials with targeted agents (see later discussion) chemotherapy resulted in disease stabilization in most patients, with very few patients experiencing disease progression after four cycles of chemotherapy. Given the poor outcome of unresectable and metastatic MPNST, histology specific trials with targeted agents have been developed. Objective responses were not observed in single agent trials with erlotinib (EGFR inhibitor), sorafenib, imatinib, dasatinib (inhibitors of several receptor tyrosine kinases), alisertib (aurora kinase inhibitor), or in combination trials of bevacizumab and everolimus (VEGF and mTOR inhibition), and ganetespib and sirolimus (HSP90 and mTOR inhibition).56,61,62 In addition to lack of objective responses, in most trials, the progression-free survival was short, with most patients experiencing disease progression after two treatment cycles. Several current treatment trials therefore define success as the clinical benefit rate, defined as objective imaging response or disease stability after 4 months (approximately four treatment cycles). Preclinical models and genetically engineered mouse models (GEMMs) of NF1-related benign and MPNST, which recapitulate the human disease, have become available and are being used to conduct preclinical trials of targeted agents (reviewed by Kim et al.).56 The hope is that these models will facilitate the selection of active agents and combination of agents for clinical trials. Concerted preclinical efforts have been joined by clinical trials coordinated through sarcoma-specific cooperative groups such as the Sarcoma Alliance for Research through Collaboration (SARC) and the Department of Defense sponsored NF Clinical Trials Consortium. These resources and infrastructure will accelerate the timely conduct and completion of clinical trials with strong scientific rationale and hopefully lead to identification of active therapies for patients with MPNST. Atypical Neurofibromas as Precursor Lesions to MPNST Atypical neurofibromas, which are defined by pathologic characteristics, such as nuclear atypia, increased cellularity, and loss of neurofibroma architecture,11 have been identified as precursor lesions for MPNST. The genetic model proposed to describe the malignant transformation from plexiform neurofibroma to MPNST through atypical neurofibroma also supports this definition based on the loss of CDKN2A observed in most cases of atypical neurofibromas.22 The recent clinical and imaging characterization of atypical neurofibromas allows for recognition of this malignant precursor lesion and through early resection possibly for the prevention to the development of high-grade MPNST.8,9 Atypical neurofibromas have a distinct nodular appearance on STIR MRI and can develop within and outside plexiform neurofibromas. The growth rate of atypical neurofibromas frequently exceeds the growth rate of plexiform neurofibromas, and in contrast to plexiform neurofibromas, most atypical neurofibromas are 282PET-FDG avid. Clinically, many atypical neurofibromas are associated with pain and palpable atypical neurofibromas are firm.9 In contrast to high-grade MPNST, atypical neurofibromas do not require resection with wide negative margins, which is surgically less challenging and results in less morbidity to patients.9,63,64 Limited data available to date suggest that atypical neurofibromas and low-grade MPNST are at low risk for recurrence after marginal resection. Given the great challenges in the treatment of high-grade MPNST, a future focus of clinical care and research should be in the identification of MPNST precursor lesions and surgical resection, if feasible, with the goal of preventing malignant transformation. A recent State of the Science meeting for MPNST recommended conducting prospective clinical and imaging studies to further define diagnostic and therapeutic strategies for atypical neurofibromas.8
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree