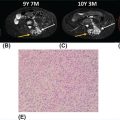
25119 Desmoid Tumor Desmoid tumors, or aggressive fibromatosis, are locally aggressive soft tissue tumors that do not have the ability to metastasize. The majority are spontaneous in nature; however, they are also seen in association with familial adenomatous polyposis. Desmoid tumors can further be divided by location (intra-abdominal, extra-abdominal, and abdominal wall) and typically present as nonmobile, fixed soft tissue masses. Computer tomography, ultrasound imaging, and magnetic resonance imaging can be utilized for diagnostic imaging and surveillance. An image-guided core-needle biopsy is necessary for diagnosis. The standard of care has been complete surgical resection. However, radiation and other systemic therapies can be used as primary, adjuvant, or neoadjuvant treatment. Treatment regimens should be individualized and require a multidisciplinary approach. This chapter discusses epidemiology, molecular pathogenesis, presentation, diagnostic approach, and therapeutic approach for desmoid tumors. Desmoid tumor computed tomography, desmoid tumors, familial adenomatous polyposis, magnetic resonance imaging, surgical resection, tumorigenesis, ultrasound imaging Adenomatous Polyposis Coli, Carcinogenesis, Fibromatosis, Aggressive, Magnetic Resonance Imaging, Tomography, X-Ray Computed INTRODUCTION Desmoid tumors, or aggressive fibromatosis, are locally aggressive soft tissue tumors that do not have the ability to metastasize. The majority are spontaneous in nature; however, they are also seen in association with familial adenomatous polyposis (FAP). Desmoid tumors can further be divided by location—intra-abdominal, extra-abdominal, and abdominal wall—and typically present as nonmobile, fixed soft tissue masses. They are more common in women and in a younger age group. Mutations leading to tumorigenesis are seen in the β-catenin or adenomatous polyposis coli (APC) genes from the Wnt/β-catenin pathway. CT, ultrasound imaging, and MRI can be utilized for diagnostic imaging and surveillance. An image-guided core-needle biopsy is necessary for diagnosis. The standard of care has been complete surgical resection. However, radiation and other systemic therapies can be used as primary, adjuvant, or neoadjuvant treatment. Watchful waiting has been used in more indolent subtypes, and a period of observation is becoming the recommendation for all types. Desmoid tumors have a high propensity for recurrence; therefore, close surveillance is essential, particularly in the first 2 years. Treatment regimens should be individualized and require a multidisciplinary approach. EPIDEMIOLOGY Desmoid tumors are a very rare form of soft tissue tumor that are locally aggressive in nature. They account for 3% of all soft tissue sarcomas, with an incidence of 2.4 to 4.3 per million per year.1 Women are two times more likely to be affected than men with the average age of 25 to 35 years (range 15–60 years).1–3 The higher propensity for women to be affected is thought to be hormonal in nature, as many stain positive for the estrogen receptor immunohistochemically.4 There is no specific racial or ethnic group preferentially affected. There are two primary subtypes of desmoid tumors, sporadic and familial. Sporadic tumors account for the vast majority of desmoid tumors, with ~90% of those encountered, equating to roughly 3.43 per million per year.5 The far less common subtype, familial, is associated with FAP. FAP is estimated to occur in one to five per 10,000 individuals and accounts for <10% of all desmoid tumors.6 Patients with desmoid tumors associated with the familial subtype have an 852 times greater risk of having a desmoid tumor than the remainder of the population.7 This is especially prevalent in the Gardner syndrome variant of FAP, which is associated with fibromas, osteomas, sebaceous cysts, and intestinal polyps. Additionally, patients with FAP who have desmoid tumors are more likely to be male, younger, and have intra-abdominal tumors.8,9 MOLECULAR PATHOGENESIS The majority of desmoid tumors arise from a mutation in the Wnt/β-catenin pathway.1 A smaller proportion arises from derangements in the APC complex that affects the same pathway.9,10 The Wnt/β-catenin pathway normally plays a role in developmental gene expression as well as regulating the amount of β-catenin available intracellularly.1 The APC complex is a kinase pathway that is inhibited by the Wnt pathway. However, when activated, APC complex phosphorylates β-catenin on threonine 41 and serine 33, 37, and 45 of the β-catenin gene on exon 3. This leads to its degradation via a proteasome within the cell, ultimately leading to decreased amounts of β-catenin.11 When inactivated by the Wnt pathway, the APC complex can no longer phosphorylate β-catenin, leading to its accumulation 252within the cell. This increase of β-catenin within the cell cytoplasm leads to translocation into the nucleus, where it plays a pivotal role in gene transcription of cyclin-D1 and c-myc. Cyclin-D1 and c-myc stimulate cell proliferation.1,12 Sporadic Subtype Roughly 95% of “wild type” desmoid tumors have mutations in the Wnt/β-catenin pathway described previously and results in majority of extra-abdominal desmoid tumors.13 CTNNB1 gene mutation is the most common that is encountered in the sporadic subtype of desmoid tumors, accounting for 85% of mutations seen.9,10,14 The CTNNB1 gene codes for β-catenin. Three different point mutations, T41A, S45F, and S45P, are seen in β-catenin.11,15 Many studies have shown higher recurrence rates and reduced 5-year disease-free survival for patients with mutations in β-catenin of CTNNB1.9,11 Specifically, the S45F mutation, has a higher rate of recurrence after surgical excision of the primary tumor; this may also be linked to anatomic location as there is a high rate of extra-abdominal desmoids with the S45F mutation.11,16,17 Multiple studies have evaluated the high rate of recurrence and specific gene markers. Current research is focusing on tailoring clinical care based on genetic markers. Other aberrations have been noted in sporadic type desmoid tumors. Trisomy 8 and trisomy 20 have been seen in up to one-third of all desmoid tumors.18,19 Roughly 64% of pediatric desmoid tumors also have mutations in CTNNB1; however, they also have mutations in AKT1 E17K in 31% of tumors, BRAF V600E in 19% of tumors, and TP53 R273H in 9% of tumors.20 Familial Subtype The vast majority of desmoid tumors are sporadic; however, patients with FAP typically have a mutation in the APC gene complex.9 Germ-line mutations in APC lead to the development of desmoid tumors in 10% to 25% of patients with FAP.12,21,22 FAP is an autosomal dominant hereditary condition caused by a mutation in the APC gene complex on chromosome 5q21-q22 that leads to hundreds of polyps within the colon.23 Owing to improvements in screening and treatment among patients with FAP, desmoid tumors have become the number one cause of death among patients who have undergone colectomy.22 The majority of patients with APC mutations are also more likely to have intra-abdominal desmoid tumors, with high preponderance for small bowel mesentery involvement.24 A young male with a primary intra-abdominal desmoid tumor should be referred for colonoscopy given the association with FAP.2 PRESENTATION Desmoid tumors typically present as painless, nonmobile masses on the trunk or arms. They can also present with bowel obstruction, or rarely, as acute bowel ischemia or functional issues with ileoanal anastomoses in patients with FAP who have previously undergone total abdominal colectomy.25,26 There are three primary anatomic locations: extra-abdominal, intra-abdominal, and abdominal wall. Molecular subtype and response to therapy vary by anatomic location. Location is an important consideration in treatment algorithm in patients presenting with desmoid tumors. Extra-Abdominal Extra-abdominal desmoid tumors occur anywhere from the head and neck, the trunk, to the extremities. Extremity desmoids occur anywhere on the arms, legs, girdles, and deep pelvis. Among extra-abdominal desmoid tumors, those in the extremity have a worse overall disease-free survival, with a 20% decreased disease-free survival rate compared to other locations.27 These lesions typically arise from musculo-aponeurotic structures. Local growth can result in limb deformation and pain. Intra-Abdominal Intra-abdominal tumors are typically associated with FAP and are primarily mesenteric in location.27 Local progression can lead to bowel obstruction, ureteral obstruction, bowel ischemia, and organ dysfunction. This anatomic site is a small subset of desmoids, accounting for only 2% to 15% of all desmoid tumors, and has up to an 88% recurrence rate.2,21,28,29 There is a 3.5% to 32% incidence among patients with FAP; however, they can occur sporadically.28 Therefore, an intra-abdominal desmoid should not be assumed to be associated with hereditary subtype until full pathologic evaluation. Any person who presents with an intra-abdominal desmoid tumor under 40 years of age should have a colonoscopy to evaluate for colon polyps and possible FAP.28,30 Intra-abdominal desmoids typically arise from within 253the small bowel mesentery. This leads to challenging surgical resection due to the risk of bowel ischemia secondary to wide local excision of the supplying mesentery.31 This can result in short gut syndrome and lifelong total parental nutrition (TPN) in some patients. Given these risks, medical management should be employed when a significant portion of the mesentery is compromised prior to undergoing surgical treatment. Abdominal Wall Patients with abdominal wall desmoid tumors typically present with a nontender, palpable abdominal wall mass. This location has the best prognosis for disease-free survival and response to therapy compared to the other locations.32,33 Wide local excision of abdominal wall tumors can lead to difficulties in abdominal wall reconstruction. This requires a multidisciplinary approach to surgical management among the surgical oncologist and reconstructive surgeon during preoperative planning.34,35 Abdominal wall desmoids are also much more common among women, and pregnant women in particular. It is thought that estrogen causes an increase in disease proliferation secondary to expression of the estrogen receptor β ligand on tumor cells; however, the exact mechanism is not understood.36,37 Despite an increase in incidence during pregnancy, it can still be managed with standard therapies including surgery or “watchful waiting.” One study showed that 14% of pregnant women who underwent watchful waiting had spontaneous regression, only 27% of patients had tumor progression, and only one patient had to undergo cesarean section secondary to her disease. However, there was a higher rate of progression of the tumor among women who were pregnant.37 There is no difference in recurrence-free survival among women with abdominal wall desmoids who are pregnant and those who are not pregnant. Pregnancy is not contraindicated in patients with history of desmoid tumors.37,38 IMAGING: DIAGNOSIS AND CHARACTERISTICS Ultrasound exam, CT, and MRI are all utilized in the diagnosis, staging, and surveillance of desmoid tumors. Ultrasound can be utilized for peripheral or abdominal wall tumors. They have variable appearance on ultrasound images and either present as well-defined hypoechoic structures or ill-defined, infiltrative masses.39 They are often identified by the “tail sign,” the tumor infiltrating along fascial planes, or the “staghorn sign,” which shows linear tumor extension.40 Ultrasound is particularly useful for pregnant women with abdominal wall tumors given the lack of radiation. It can also be used to serially follow extremity/abdominal wall desmoids. Typically, cross-sectional imaging is the initial step in diagnosis of desmoid tumors. It allows for further delineation of tumor size, surrounding anatomic involvement, and invasion into any adjacent structures. CT scan with contrast is the preferred imaging modality for intra-abdominal desmoid tumors. They usually appear as poorly defined masses with variable contrast attenuation. The amounts of collagen and myxoid components dictate the appearance on CT.39,41 The more myxoid component, the more hypodense the lesion will appear. The more skeletal muscle, collagen, and fibrotic components, the more hyperdense the lesion will appear.40 However, it is very rare to have necrosis or calcifications seen on CT imaging. Intra-abdominal desmoid tumors seen with FAP typically have extensions with radiating spicules into the mesentery and which can help delineate any other organ impingement or bowel obstruction.42 MRI, specifically T2-weighted imaging, is the modality of choice for evaluating abdominal wall and extra-abdominal desmoid tumors. It most commonly appears as low intensity of T2-weighted images and hyperintense on T1-weighted images.40 Another typical finding on MRI is the “band sign,” which is low signal, nonenhancing bands within the tumor.40,43 Similar to CT imaging, tumors can have a heterogeneous appearance based on myxoid and collagenous components. It is also rare to see necrosis.43 MRI is also useful in the assessment of desmoid tumors among pregnant women. DIAGNOSTIC APPROACH In order to diagnose desmoid tumors, an image-guided core-needle biopsy is recommended. This can be done via ultrasound for peripheral tumors or via CT-guided biopsy for intra-abdominal or deep pelvic tumors. Pathologic examination shows poorly circumscribed, infiltrating lesions that have an abundance of spindle cells within collagen. They also have low cellularity and proliferation of fibroblasts. Immunohistochemical staining is positive for muscle-specific actin (MSA) and smooth muscle actin (SMA) and negative for desmin, h-caldesmon, and S-100.1 Additionally, 40% to 91% stain positive for 254β-catenin; however, some advocate that a better diagnostic marker is a mutational analysis of CTNNB1 for diagnosis.44,45 Differential diagnoses include scar tissue, low-grade fibromyxoid sarcoma, nodular fasciitis, myofibroma, Gardner fibroma, and collagenous fibroma. THERAPEUTIC APPROACH The primary treatment for desmoid tumors is surgical resection. Nonsurgical therapies include hormonal therapy, chemotherapy, tyrosine kinase inhibitors (TKI), radiation, and “watchful waiting.” The watchful waiting approach is reasonable because approximately 20% of tumors undergo spontaneous regression. Treatment regimens are described in detail in the following sections. Local control still remains difficult, despite which modality used, with recurrence rates between 24% and 77%.46,47 Table 19.1 summarizes response and recurrence rates of the various treatment modalities. Surgery Standard treatment for desmoid tumors is wide local excision to negative margins. Intraoperatively, the goal is wide local excision with 1-cm margins when possible.48 However, based on location and size of the tumor, this can lead to significant morbidity and even death. Multiple studies have evaluated the recurrence rate for margin negative (R0) disease and recurrence potential. Patients with R0 resection have 5-year recurrence rates ranging from 16% to 25%.27,49,50 Five-year recurrence rates increase with microscopically positive margins (R1) on final pathology, ranging from 19% to 48% for R1 resection.27,49,51,52 Given the high rate of recurrence after surgical resection, several studies have evaluated prognosis with regard to R0 versus R1 resection. Some studies have shown improved prognosis with microscopically negative margins,50,53,54 while others have shown minimal to no prognostic benefit to R0 resection.51,55 Given these findings, the goal of surgery should be R0 resection. However, this should be limited based on organ compromise or severe limb/disfigurement from resection because the debate of margin status on prognosis is still in question. Indeed, some surgical series show that R0 margins are achieved in only a minority of patients. 255Frozen section has been used intraoperatively to help determine margin status.46,48 However, desmoid tumor is hard to distinguish from scar tissue on frozen section. Furthermore, it does not improve the rate of recurrence.46 Therefore, frozen section analysis intraoperatively is not recommended. As stated previously, excision of desmoid tumors can be difficult based on size and location. When addressing abdominal wall tumors, these large masses can leave significant abdominal wall defects, requiring a multimodality approach to repair. At our institution, we commonly involve plastic surgery for assistance in coverage of large defects requiring an array of soft tissue transfers and flaps. Mesh is also commonly employed. Mesh is safe to use in reconstruction of abdominal wall defects after resection of desmoid tumors, with low primary tumor recurrence and long-lasting abdominal wall repairs.33 For the intra-abdominal tumor location, it is important to close the peritoneum, even with staged procedures, in order to prevent fistula tract formation. Mesh is also a viable option for closure of the abdominal wall after intra-abdominal tumor resection. This location can present a particularly difficult problem when a significant portion of the mesentery is compromised or the tumor is in proximity to the origin of the superior mesenteric artery. Again, a multidisciplinary approach can be utilized with vascular surgery for involvement in bypass procedures of the superior mesenteric artery in order to obtain R0 resection.56 Surgical management for extremity desmoid tumors should focus on limb salvage. If the tumor is not resectable without limb loss, it is reasonable to treat the primary tumor with other modalities or the addition of adjuvant therapy with positive margins. Multiple studies have shown that limb salvage with adjuvant treatment modalities is a safe option for patients with extremity desmoid tumors.57–59 Therefore, standard of care for extremity desmoid tumors is limb salvage, with R1 resection if necessary followed by adjuvant systemic therapy or radiation as indicated. Isolated limb perfusion has also been utilized for extremity tumors that are unable to achieve R0 resection. Limb perfusion using tumor necrosis factor-α (TNF-α) and melphalan-based isolated limb perfusion (TM-ILP) has shown response rates as high as 75%. However, these are associated with the known side effects of epidermolysis and intractable pain.60,61 Amputation does remain an option for patients who do not tolerate local therapy, have continued growth of the primary tumor, desire primary amputation, or have a poor functional status at baseline. Hormonal and Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) Both NSAIDs and hormonal therapy are commonly employed as adjuvant therapy for desmoid tumors. Given the propensity for desmoid tumors to occur in females and that they have been found to stain positive for the estrogen receptor, antiestrogen agents are used; however, the exact mechanism is unknown.2,62–64 Hormonal therapy is typically employed as adjuvant therapy and for recurrence of desmoid tumors. They have a low overall side effect profile and are typically tolerated well by patients. Most commonly, tamoxifen and raloxifene are used as first-line antiestrogen agents with partial response rates as high as 40% to 51%.21,65 Other hormonal agents that have to be used include megestrol, progesterone, goserelin+tamoxifen, and testolactone. With regard to NSAIDs, sulindac is the most commonly employed NSAID for systemic treatment, but indomethacin is also used.66 However, response rates to NSAIDs alone are low, ~27%.21 Given the low response to individual therapy with antiestrogen or NSAIDs alone, combination therapy has been used. When used in combination, response rates were as high as 85% in one study, for both patients with sporadic type and FAP-associated tumors, defined as stable disease or regression.67 Furthermore, these agents were able to be discontinued with only one long-term recurrence. Given these sustained findings, high-dose NSAID and antiestrogen therapy can be applied in patients who were not resectable or had R1 disease. Use for many months may be required before there is any relief of symptoms or reduction in disease.67–69 Celecoxib (Celebrex) and other cyclooxygenase-2 (COX-2) inhibitors have been used for management of desmoid tumors, either as single therapy or in combination with other agents.70–72 COX-2 256inhibitors are thought to independently target the Wnt/β-catenin pathway that is also affected by desmoid tumor pathogenesis.73 However, the literature to support its use is sparse, with case studies or small pilot trials. Further work needs to be done to address this possible therapeutic agent for the treatment of desmoid tumors. Radiation Therapy When tumor resection would lead to functional deficits or is in a location that is otherwise unresectable, radiation has been shown to be effective treatment for desmoid tumors. Rates of local control are ~80% for disease that is not amenable to surgical resection.74,75 The European Organisation for Research and Treatment of Cancer (EORTC) performed a Phase II clinical trial, evaluating patients with unresectable disease who underwent a standardized radiation regimen with local control in ~80% of patients. Complete response was low, with only 13% of patients with complete response to radiation therapy; partial response was noted in 36.4%, and stable disease was noted in 40.9% of patients.74 It is important to recognize that the response to radiation therapy can occur slowly over the years after completion of treatment. There is interest in combining systemic and radiation therapy for unresectable desmoid tumors. A small study evaluated imatinib with radiation therapy with promising results for local control.76 Radiation therapy can be used both in the neoadjuvant and adjuvant settings as well as definitive therapy for desmoid tumors that are unresectable. Two large meta-analysis studies have evaluated surgery without adjuvant radiation and adjuvant radiation therapy after surgery. One study saw superior local control of tumor for those who underwent radiation with surgery compared to surgery alone (75% vs. 68%, respectively).77 Another study evaluated patients with R1 resection who were treated with and without adjuvant radiation therapy. The overall recurrence of desmoid tumors was higher with R1 resection, but there was an overall improvement in recurrence rate with adjuvant radiation for both primary tumors and recurrence.78 However, there have been no prospective, randomized control trials evaluating radiation as adjuvant therapy for desmoid tumors. The rarity of desmoid tumors makes it a challenging disease in which to conduct randomized clinical trials. Furthermore, the radiation doses used in the past were variable and based on institutional practices, making evaluation of these studies difficult. Current National Comprehensive Cancer Network (NCCN) guidelines suggest 50 Gy in conventional fractionation for adjuvant radiation and 50 to 56 Gy for treatment of unresected disease. Smaller, retrospective studies have shown no benefit for surgery alone compared to surgery with radiation.79 Radiation can also have significant side effects including skin irritation, edema, and fibrosis, although these are generally mild-moderate in modern series,74 and also carries the risk of a radiation-associated malignancy. Given these issues, it is important to employ a multidisciplinary approach when evaluating patients for radiation therapy. Neoadjuvant radiation therapy has also been evaluated; however, like adjuvant therapy, there have been no randomized prospective studies. Furthermore, the literature is sparse and the available studies are small and not standardized. There is a proposed benefit to improve local control and to bridge patients to resectable disease. Similar to the other adjuvant treatments described, chemotherapy has been used in conjunction with radiation with response rates as high as 90%.80,81 Chemotherapy Systemic chemotherapy agents are typically used for unresectable or recurrent disease with response rates ranging from 30% to 80%, depending on agent and regimen used.21,69,82,83 Agents include methotrexate, vinblastine, cisplatin, and (most commonly) anthracyclines. Chemotherapy is usually second-line systemic therapy given the side effect profile. However, if the disease is rapidly progressing, unresectable, or encroaching on critical adjacent structures, it can be used as first-line therapy.83–85 Similar to NSAID and hormonal therapies, it can take multiple months prior to seeing any effect from treatment.83 First-line chemotherapy agents are anthracycline-based regimens due to high response rates and longer periods of progression-free survival (PFS). One study evaluated anthracycline-based chemotherapy regimens compared to other forms of systemic therapy (i.e., NSAIDs, antiestrogens, and imatinib). They found a 54% response rate with anthracycline-based chemotherapy compared to only 12% with the other treatment modalities (p = .0011), with a higher PFS for patients who did not have extremity location desmoid tumors.83 Others have shown increased PFS of 74 months while on anthracycline-based therapy.82 However, a trade-off is the potential cardiotoxic side effects of anthracycline-based treatment that can make this a less favorable option for some patients. Studies have also evaluated methotrexate with vinblastine for low-dose chemotherapy regimens with promising results.86,87 However, these studies are limited given the small sample size. 257Tyrosine Kinase Inhibitors TKIs including imatinib, sunitinib, sorafenib, and pazopanib have been used for treatment of desmoid tumors with response rates of approximately 20% and minimal toxicity. Multiple Phase II clinical trials have been performed evaluating imatinib for treatment of desmoid tumors. They have shown PFS in ~65% of all patients. However, the overall response rate was low, ranging from 6% to 16% with the higher response rate seen in patients receiving higher doses of imatinib. Side effects for these trials was overall low.88–90 Sunitinib has also been used for treatment of desmoid tumors. It appears to be more potent than imatinib with small studies showing that sunitinib may be beneficial when imatinib was not.91 A Phase II clinical trial showed that roughly one-fourth of all patients responded to therapy, of which, three-fourths had a 2-year PFS. However, there was significant adverse reactions to therapy with bleeding, bowel perforation, and fistula to tumor for intra-abdominal mesenteric desmoid tumors.92 Patients being treated with sunitinib need to be counseled on the possible adverse reactions. Sorafenib has been used for treatment of desmoid tumors; until recently it has only been studied in Phase II studies. These studies revealed partial response rates of 25% with sorafenib, with stable disease in 70% of patients.93 A recent Phase III clinical trial of sorafenib has reported results on the first 75 patients; the response rate was 33% and PFS doubled over the placebo arm.94 Sorafenib has also been used in conjunction with celecoxib in case studies with major responses in patients who had recurrent disease.70 To date, the use of pazopanib has been limited. Only one small case report has shown improved symptoms and response to pazopanib.95–97 Similar to sorafenib, results of a recently completed clinical trial were reported but have not been finalized; improved responses and PFS were seen compared to methotrexate vincristine.98 Final results of these studies are awaited to evaluate the overall benefit of these TKIs in the treatment of desmoid tumors. Novel Therapy There are several new therapies investigating recently discovered pathways and targets in desmoid tumors. A Phase I trial that is investigating PF-03084014, an oral reversible γ-secretase inhibitor, is currently ongoing. This γ-secretase inhibitor interacts with the Notch pathway. Initially developed for Alzheimer’s treatment to inhibit β-amyloid accumulation, it is also being investigated in desmoid tumors. The Notch pathway involves four surface receptors that function in most cases as an oncogene. These receptors are particularly high on stromal cells, the exact mechanism with regard to desmoid tumors is unknown, but is likely due to cell cycle arrest.99,100 The γ-secretase inhibitor has shown tumor response in desmoid tumor in ~30% of patients and additional stable disease in ~30% of patients.101 The side effect profile is also low and well tolerated, with nausea, vomiting, hypophosphatemia, and hypertension as the most common side effects.99 This shows promise for new novel treatment of desmoid tumors, with ongoing studies looking at the efficacy of γ-secretase inhibitor efficacy on a larger cohort of patients.99 Rhamm is a protein seen in wound healing and neoplastic processes that is expressed in high levels on desmoid tumors. Small animal models have shown Rhamm associated with cell–cell contact and proliferation.102 It may be a new target in the treatment of desmoid tumors. Another new therapy for desmoid tumors is targeting hyaluronan (HA), a glycosaminoglycan on mesenchymal cells. Immunochemistry has shown that HA plays a role in cell signaling and tumor proliferation. This interaction may lead to targeted therapy that inhibits this interaction.103 Watchful Waiting There has been a growing body of evidence that advocates for surveillance alone of desmoid tumors, or “watchful waiting.” It stems from the high rate of recurrence after surgery, the observation of spontaneous regression in some patients, and high morbidity seen with some tumor resections. This approach includes active, careful surveillance of patients on a regular schedule with appropriate imaging based on location for 2 months consecutively, then 3 months for 1 year, followed by 6 months for 5 years, and then yearly for an indefinite time period.68 The best candidates for the watchful waiting approach are those with no symptoms and are not related to any critical anatomic structures. More aggressive management may be more appropriate for those tumors in close relation to such structures. Watchful waiting was initially advocated in Europe and has since been more widely employed in the United States. A study in the European Journal of Surgical Oncology in 2008 showed that patients who were managed with surgery (R0 resection) versus those patients under surveillance with medical therapy had the same event-free survival rate of 65%. The only difference between the two groups in regard to improved event-free survival was quality of resection and location of tumor.32 This led to 258a wider study to look at patients being treated with systemic therapy compared to no therapy. They found that there was no difference in PFS among the groups (49.9% vs. 58.6%, respectively).37 The majority of recurrences were seen in the first 2 years,53 advocating for the highest surveillance and monitoring in those early years. Some institutions are now using “watchful waiting” as first line after diagnosis of desmoid tumor and only move to other treatment modalities if the tumor is an aggressive phenotype.28,68 FOLLOW-UP AND SURVEILLANCE As stated previously, the highest rate of desmoid tumor recurrence is seen within the first 2 years.53 Patients should undergo history and physical exams along with appropriate imaging, based on location every 3 to 6 months for the first 2 to 3 years and then annually after that time.104 PROPENSITY TO METASTASIZE Unlike other soft tissue tumors, desmoid tumors lack the ability to metastasize to other locations in the body, although multifocal tumors can occur, particularly in the extremities.105,106 However, as discussed previously, they act aggressively, invading surrounding structures and recur locally even with microscopic and gross margin negative resections. THERAPEUTIC APPROACH FOR RECURRENT DISEASE Desmoid tumors have a very high recurrence rate, ranging from 19% to 77%.77 For recurrent disease, surgical resection (as described previously) is standard of care if surgically resectable. However, radiation and other systemic treatment modalities can be utilized for recurrent disease. Patients with recurrent desmoid tumors should be evaluated by a multidisciplinary team and should be treated with the modality that is the most appropriate based on location, tumor biology, and associated structures involved. No single therapy has been shown to be the most effective for recurrent disease. SUMMARY Desmoid tumors are characterized by their infiltrative nature and propensity for recurrence. For asymptomatic patients, a period of watchful waiting to characterize the individual disease process is useful. This must be balanced against potential critical organ resection if there is progression. Particularly mesenteric desmoids should be considered for nonsurgical therapies as the majority involve a significant portion of mesenteric vasculature that can lead to intestinal compromise, fistula, and abdominal wall disasters with resection. For extremity lesions, imaging is invaluable in determining total extent of disease as often these lesions have long tails and the palpable mass on exam is only a small portion of the entire lesion. Sparing patients large morbid resections should be the strategy with initial resection, which should aim for gross total resection because recurrence rates are similar for microscopically positive or negative margins.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree