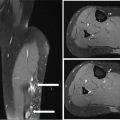
32925 Myxoid Liposarcoma Myxoid liposarcoma (MLS) accounts for about 30% to 35% of liposarcomas and approximately 10% of all adult soft tissue sarcomas. According to the most recent World Health Organization classification, MLS can be morphologically divided into high and low grades. MLS has a peculiar natural history: In contrast with other sarcoma subtypes, which primarily metastasize to the lung, MLS tends to spread to serosal membranes, the abdominal cavity, soft tissues, and bone. Regarding the treatment of this histotype, the mainstay for localized disease is surgery, alone or in combination with radiation therapy. Chemotherapy is used in patients with unresectable advanced disease. When recurrence develops, surgery is an option, although the appropriate candidates must be carefully selected. Multimodality therapy is frequently used in these situations. For patients with unresectable and/or metastatic disease, the systemic therapy option is chemotherapy. This chapter reviews diagnostic approach and common anatomic location of primary MLS, as well as therapeutic approach for metastatic disease. abdominal cavity, bone, chemotherapy, metastatic disease, multimodality therapy, myxoid liposarcoma, radiation therapy, surgery, systemic therapy Abdominal Cavity, Bone and Bones, Combined Modality Therapy, Drug Therapy, General Surgery, Liposarcoma, Myxoid, Neoplasm Metastasis, Radiotherapy INTRODUCTION Myxoid liposarcoma (MLS) accounts for about 30% to 35% of liposarcomas and approximately 10% of all adult soft tissue sarcomas (STSs). According to the most recent WHO classification, MLS can be morphologically divided into high and low grades. The degree of aggressiveness depends on the percentage of the hypercellular component within the tumor.1,2 These neoplasms harbor two characteristic chromosomal translocations, namely a t(12;16)(q13; p11), resulting in the fusion of TLS–DDTI3 genes, and a rarer t(12;22)(q13;q12), resulting in the fusion of EWS and DDTI3 (Figure 25.1). So far, different molecular variants of the TLS–DDIT3 fusion have been described, although with an undefined impact on clinical outcome.3,4 MLS has a peculiar natural history: in contrast with other sarcoma subtypes, which primarily metastasize to the lung, they tend to spread to serosal membranes, the abdominal cavity, soft tissues, and bone.5 Regarding the treatment of this histotype, the mainstay for localized disease is surgery, alone or in combination with radiation therapy (RT). Unfortunately, despite adequate local treatment, approximately 40% of patients relapse, and median survival following first documented metastasis is about 2 years.6 Chemotherapy is used in patients with unresectable advanced disease.7–9 A higher sensitivity to standard chemotherapy and RT compared to the other STS histologic types has been suggested, but long-term tumor control is far from optimal.10,11 When recurrence develops, surgery is an option, although the appropriate candidates must be carefully selected. Multimodality therapy is frequently used in these situations. For patients with unresectable and/or metastatic disease, the systemic therapy option is chemotherapy. Several newer drugs including immunotherapy are under investigation. MLS is a young adult disease, with the age at presentation on average a decade younger than that for the other histologic subtypes of liposarcoma. It has a peak of incidence in the fourth and fifth decades of life, and although very rare, it is the commonest form of liposarcoma in patients younger than 20 years of age. There is no gender predilection.2 MOST COMMON PRESENTING SYMPTOMS MLS typically occurs as a large painless mass within the deep soft tissues of the limbs while, in contrast with the well-differentiated group, the retroperitoneal location seems to be exceptional.2 A diagnosis of primary MLS in the retroperitoneum should be regarded with suspicion, as most such cases represent either metastatic MLS or well-differentiated/dedifferentiated liposarcoma with myxoid stromal change. Metastatic sites that could cause presenting symptoms include bone (pain or fracture), lung (dyspnea), and fatty tissue (peritoneal, pericardiac, paratracheal, etc). DIAGNOSTIC APPROACH Accurate diagnosis of MLS including tumors arising in the extremity and trunk, usually consists of MRI. However, there are pitfalls, and expertise from a radiologist familiar with the disease is critical. For patients with suspected or confirmed MLS, staging should be done with a contrast-enhanced chest and abdomen CT scan and MRI of the vertebral column, as bone, mediastinum, and abdominal 330cavity are the most common metastatic sites.6 The roles of PET or whole-body MRI remain to be determined. MLS indeed may or may not have a metabolic activity on PET scan and whether this correlates with disease aggressiveness is currently under investigation. Whole-body MRI may detect earlier metastatic disease, particularly to the bone. However, the impact of early metastases detection is far from being demonstrated as critical for cure. FIGURE 25.1 Specific chromosomal translocations of myxoid liposarcomas. CHOP/DDIT3, fusion gene; FUS/TLS, gene; EWS, gene. Ultimately, to establish a diagnosis, the gold standard is histologic examination of tumor tissue. In the preoperative setting, tissue is most commonly obtained through core-needle biopsy. In the past, MLS was separated from round cell liposarcoma (RCL); however, strong morphologic as well as genetic data have led to the classification of myxoid and RCL together as part of a morphologic continuum, which includes hypercellular neoplasms composed of oval to round neoplastic cells. Cytogenetics and molecular genetics have greatly contributed to such a conceptual reappraisal by demonstrating that both myxoid and RCLs contain the same genetic abnormalities (that is, balanced translocations), most commonly t(12;16)(q13;p11), which fuses the DDIT3 (CHOP) gene on 12q13 with the FUS (TLS) gene on 16p11.2 The classic myxoid variant shows low cellularity, conspicuous vascular network, and myxoid stroma, whereas the cellular variant shows high cellularity, made up of closely packed roundish cells, little or no intervening stroma, and a capillary pattern not easily visualized. Diagnosis of a high-grade variant requires the presence of a cellular component exceeding 5%.1 This amount is of prognostic relevance because within the myxoid liposarcoma (MLS) spectrum, the 5-year survival rate varies between 20% and 70%, and the cellular variant falls in the shortest survival time. However, the 5% cutoff is far from being perfect to distinguish two separate entities and may lead to under- or overestimation of risk. The risk increases continuously with the rate of hypercellularity rather than by a binary classification. In summary, the higher the cellularity, the greater the risk of recurrence, metastasis, and death. As the extent of hypercellularity predicts the risk of metastatic spread, extensive sampling of the surgical specimen is critical. Microscopically, pure MLS is remarkably hypocellular, featuring a bland spindle cell proliferation set in an abundant myxoid background. Lipoblasts are most often monovacuolated and tend to cluster around vessels or at the periphery of the lesion. The most helpful morphologic clue is the presence of a thin-walled, capillary-sized vascular network, organized in a distinctive plexiform pattern. High-grade MLS (formerly RCL) is defined by the presence of hypercellularity. Adipocytic differentiation in pure RCL is rarely appreciable, but in such circumstances the presence of S-100 immunopositivity may be diagnostically helpful.2 MLS is characterized by two main karyotypic aberrations: more than 95% of cases carry a specific t(12;16)(q13;p11), which fuses the DDIT3 (CHOP) gene on 12q13 (a member of the CCAAT/enhancer binding protein family involved in adipocyte differentiation), with the FUS (TLS) gene on 16p11. Approximately 5% of MLSs harbor a t(12;22) (q13;q12), which fuses DDIT3 with EWSR1 on 22q12. PIK3CA mutations have also been detected in a subset of cases; this may represent a valuable additional target for future molecularly driven therapies.12 FIGURE 25.2 Case example of high-grade myxoid liposarcoma treated with five cycles of epirubicin and ifosfamide and concomitant radiation therapy. The pathologic specimen after the surgery showed a complete pathologic response. (A) MRI of a high-grade myxoid liposarcoma, (B) MRI with a radiological response, (C) pathologic specimen after surgery. COMMON ANATOMIC LOCATION OF PRIMARY MLS MLS occurs with predilection in the deep soft tissues of the extremities, and in more than two-thirds of cases arises within the musculature of the thigh. MLS rarely arises primarily in the retroperitoneum or in subcutaneous tissue. Surgery is the mainstay of treatment in patients with localized disease, alone or in combination with RT. To optimize local control, RT eventually in combination with systemic chemotherapy given before surgery may be of benefit, in order to obtain a cytoreduction and to improve the prognosis.6 In particular, it has been repeatedly observed that the presence of hypercellularity or round cell differentiation is associated with worsening of prognosis. The 90% 5-year survival rate of MLS decreases to 20% for patients with RCLs.6 Thus, for patients with high-grade MLS, even after complete resection, there remains a high risk for recurrence and death. Therefore, high-grade MLS may benefit from the above-mentioned multimodality treatment, either before or after surgery7 (Figure 25.2). Overall, the utility of systemic treatment and the sequence of therapy for each patient with MLS should be discussed within a multidisciplinary conference at a sarcoma reference center. Neoadjuvant therapy (RT and/or systemic treatment) may facilitate resectability in borderline cases, especially if tumor shrinkage can be achieved and improve the prognosis. Neoadjuvant combined chemotherapy-RT has been extensively explored in localized STS. A recent randomized trial showed a benefit of neoadjuvant anthracycline and ifosfamide doublet (AI) versus a histology-driven chemotherapy in terms of overall survival (OS) and recurrence-free survival (RFS), thus pointing to efficacy of neoadjuvant treatment as such in a subset of STS. In the MLS stratum of this trial, the interim analysis showed the noninferiority of trabectedin, with a much better toxicity profile. This study is still recruiting in the MLS stratum and is expected to complete the accrual in a year.13 Trabectedin was recently shown also to be combinable with RT in patients affected by locally advanced MLS, with promising results.14 If trabectedin proves to be equivalent to AI, its use alone or in combination to RT may well become the preferred approach in treatment of localized high-risk MLS. RECOMMENDED FOLLOW-UP Given the risk of recurrence, close surveillance is important in patients with MLS. Although consensus recommendations (National Comprehensive Cancer Network [NCCN], European Society of Medical 332Oncology [ESMO]) exist for surveillance in STSs in general, there are no validated guidelines specific for MLS.15 Most sarcoma centers recommend initial contrast-enhanced CT of the chest and abdomen at 3 to 4 months after surgery, and every 4 to 6 months for at least 2 to 3 years and up to 5 years. A similar schedule of interval imaging should be followed with contrast-enhanced MRI. Whole-body MRI has been suggested as an alternative, to offer patients to be staged with a single modality, without radiation exposure, and with high sensitivity in detecting recurrences of this disease at all sites, including bone. For superficial tumors, ultimately MRI may be replaced with ultrasound imaging. Surveillance should be modified based on personalized risk and anticipated pattern of recurrence. Late recurrences can occur, particularly in low-grade MLS. When recurrence develops (usually detected by surveillance imaging), decision-making and overall management can be complex and multimodality therapy is frequently used. Surgery is always considered and is ultimately performed in most patients with a localized recurrence. Currently, there are no laboratory tests that are useful to detect recurrence in MLS. PROPENSITY TO METASTASIZE MLS is prone to recur locally, and one-third of patients develop distant metastases, but the probability of developing distant metastases depends on the histologic grade. In contrast to other types of liposarcoma or other sarcomas of the extremities, MLS tends to metastasize to unusual soft tissue (such as retroperitoneum, opposite extremity, axilla, etc.) or bone (with predilection to spine) locations, even before spread to the lung.5 In a significant number of cases, MLS patients present clinically with synchronous or metachronous multifocal disease. This unusual clinical phenomenon most likely represents a pattern of hematogenous metastases to other sites by tumor cells seemingly incompetent to seed the lungs. The extent of hypercellularity predicts the risk of metastatic spread. THERAPEUTIC APPROACH FOR METASTATIC DISEASE Treatment options for patients with unresectable and metastatic disease are limited. In STS in general, front-line treatment for unresectable/metastatic disease is usually systemic therapy with doxorubicin, alone or in combination with ifosfamide, with a reported objective response rate (ORR) in the range of 20% to 40%.7–9 The combination of doxorubicin and ifosfamide is superior to doxorubicin alone. MLSs are known to be particularly sensitive to chemotherapy compared with other STSs,9,10 especially to anthracycline-based combinations and trabectedin. The response rates to the front-line combination of an anthracycline and ifosfamide is approximately 65%. On the other hand, the expected response rate of MLS to trabectedin, approved for second-line therapy, is around 80%, with a mechanism of action thought to be due to binding to the fusion transcript and target genes.16–19 The extra activity of trabectedin in MLS was observed quite late in the development of the drug in sarcomas. In 2007, we were able to report on 51 patients with MLS who had been treated on a compassionate basis at five institutions in Europe and the United States. In this series of MLS, the response rate according to dimensional criteria was approximately 50%, and the 6-month PFS was about 80%.20 Thus, the drug showed a major antitumor impact, in a heavily pretreated patient population, which was substantially different from what observed in other STS, including the most sensitive leiomyosarcoma and well-differentiated/dedifferentiated liposarcoma. At a longer follow-up, we updated this series regarding the subgroup of 32 patients treated in Milan. The median PFS was confirmed to be prolonged, that is, 17 months. Treatment was continued for a long time in several patients. Indeed, long progression-free intervals were observed also in patients who discontinued their treatment with trabectedin after achieving an objective response or stable disease. When they progressed, a re-challenge with trabectedin induced further responses, with relatively long progression-free intervals.21 Some patients underwent surgery of residual disease, following the practice of surgery of extrapulmonary metastases in low-grade MLS, given the relatively indolent course of disease. However, the added value of metastasectomy is doubtful when the medical therapy has been ineffective. An intriguing clinical observation was that tumor responses could be nondimensional initially, before giving rise to tumor shrinkage at a later time. The hallmark in these cases is a decrease in tumor contrast enhancement and radiodensity.21 Fortunately, the sarcoma clinical community had learned the lesson deriving from gastrointestinal stromal tumors (GISTs) treated with targeted therapies, so we were ready to appreciate this type of response.22,23 Clinicians should be aware of this pattern of tumor 333response in MLS, to avoid mistakes. However, this also led us to suspect that a different mechanism of action could be in place, in other words that such a pattern of tumor response, which we are used to observe with molecularly targeted therapies, could point to a kind of “targeted” mechanism of action also for a chemotherapeutic agent like trabectedin. Preclinical studies performed in an MLS cell line indicate that trabectedin modulates transcription, presumably interfering with transcription factors that are deregulated in some sarcomas. In particular, MLSs are marked by the fusion gene FUS-CHOP. In the presence of trabectedin, it is transcribed normally, but is not able to activate the transcription of target genes. Thus, the mechanism of action of trabectedin is highly selective, with a depression in the expression of genes that are crucial for the late phase of adipocytic differentiation.24 Recently, another marine-derived drug, eribulin, was shown to be effective in metastatic adipocytic sarcomas, with a significant improvement in OS, which led to its approval by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA).25–27 However, its activity in MLS compared to pleomorphic and well-differentiated/dedifferentiated liposarcoma is still not defined. No other agents commonly used for treatment of advanced STS (e.g., gemcitabine, dacarbazine, and pazopanib) have been demonstrated to be active in MLS. Continuous infusion, high-dose ifosfamide (icHDIFX), which is another treatment option active in advanced well-differentiated/dedifferentiated liposarcoma patients, seems not to be active in MLS.28 In conclusion, the more active drugs for advanced MLS are anthracyclines-based regimens (usually ifosfamide) and trabectedin. Other treatment options are under investigation, including immunotherapy such as checkpoint inhibitors and cellular therapy. Indeed, most patients with MLS have homogeneous expression of the highly immunogenic tumor-associated antigen NY-ESO-1 or MAGE. Furthermore, while these patients have rare NY-ESO-1 or MAGE-specific T cells circulating in their blood, they lack a robust inflammatory response and generally have relatively few T cells infiltrating the tumor.29 Agents and strategies with the ability to induce a robust endogenous tumor-specific T-cell population have the potential to dramatically activate the immune response against this neoplasm and are under investigation. SUMMARY In conclusion, MLS comprises a spectrum of disease with a spectrum of aggressivity that parallels the percent cellular component. Localized disease, especially for high-grade MLS, requires a multidisciplinary approach as the combination of RT and chemotherapy are recommended in the preoperative setting. The exceptional activity of trabectedin in this neoplasm encourages exploration of the efficacy of the drug in first line and in the preoperative setting. Unfortunately, up to now, even if MLS seems to be sensitive to chemotherapy, no other options are active in the advanced setting beyond the use of anthracycline-based combinations (usually ifosfamide) and trabectedin. Treatment options using adaptive immunotherapy look promising and are under investigation.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree