Key points
- •
Patients with cirrhosis are at highest risk of developing hepatocellular carcinoma (HCC) and screening with sonography every 6 months is recommended.
- •
Early-stage HCC should be treated by resection, liver transplantation, or ablation. The best modality depends on tumor size and location, number of tumors, liver function status, future liver remnant, organ availability, as well as patient’s fitness for surgery.
- •
Curative anatomic liver resection is the optimal treatment for HCC in noncirrhotic patients and in selected patients with Child-Pugh Class A cirrhosis, with small solitary tumors. Laparoscopic liver resection has benefits in selected cases.
- •
Patients who are not suitable for resection and meeting the Milan criteria should be considered for liver transplantation (living or cadaveric). Bridge therapy with locoregional treatments should be utilized during transplant evaluation and waiting time.
- •
Patients who are not surgical candidates should be treated with loco-regional approaches such as ablation and transarterial therapies. Transarterial therapies include bland embolization, chemoembolization, and radioembolization.
- •
Inoperable patients may be offered systemic treatment with antiangiogenic drugs, such as sorafenib. The role of systemic treatment in an adjuvant setting remains to be determined. Inclusion in trials of new drugs should be encouraged.
- •
Because of multiple treatment options, multidisciplinary team/conference is the indispensable platform for best comprehensive care.
Introduction
Hepatocellular carcinoma (HCC) is the most common primary malignancy of the liver. Surgical management of this deadly cancer remains a challenge for all involved in the care of patients diagnosed with it. It is the second most common cause of cancer-related death in the world in men and sixth in women. , The mortality per year in HCC is close to its incidence worldwide, emphasizing the high fatality rate of this aggressive disease. Liver cirrhosis, irrespective of the etiology, is an established major risk factor for HCC. Chronic hepatitis B and C are recognized as the main risk factors for HCC, with risk being escalated in the presence of coinfection with hepatitis B virus (HBV) and hepatitis C virus (HCV).
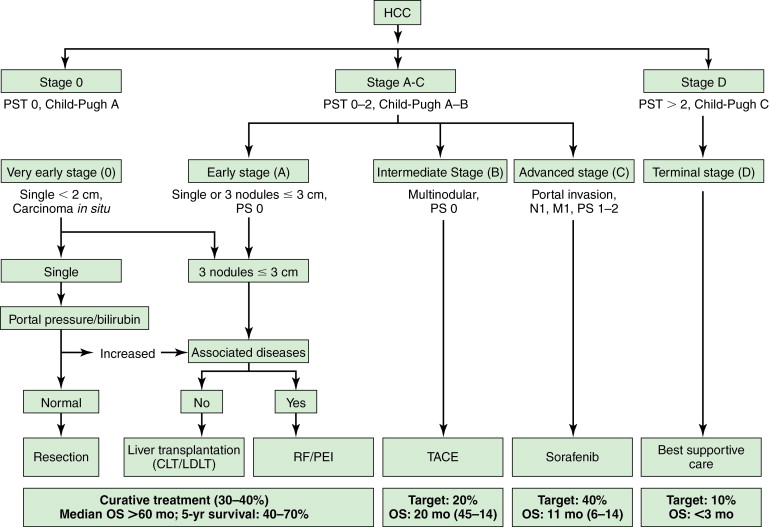
The Barcelona Clinic Liver Cancer (BCLC) classification is one of the widely used and accepted classification; it stratifies patients according to outcome, avoids the use of a single scoring system for all patients, and simultaneously links it with treatment strategy ( Figure 7-1 ). The BCLC classification is constructed in the setting of cohort and randomized controlled studies; it has also been validated in different settings and establishes treatment recommendations for all stages of HCC. , Patients with early-stage HCC are treated by resection, liver transplantation, or ablation, and prognosis can be refined for all these procedures according to different variables. It considers variables related to tumor size, liver function status, physical status, as well as cancer-related symptoms.
Treatment strategy
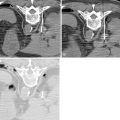
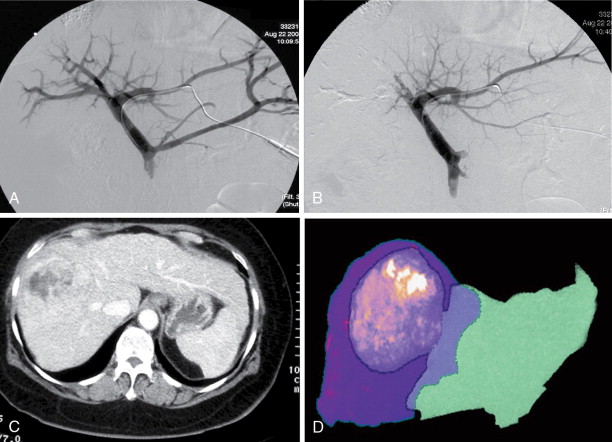
Surgery, including surgical resection and liver transplantation, is currently the mainstay and treatment of choice for HCC. In general, the principles of surgery for HCC after the patient is evaluated to be medically fit for general anesthesia and major surgery are as follows. Liver resection is indicated as a potentially curative procedure if the patient has an adequate liver function (generally Child-Pugh Class A without portal hypertension) and the tumor is resectable with an adequate future liver remnant of at least 20% in a noncirrhotic liver or at least 30%–40% in patients with Child-Pugh Class A cirrhosis. For patients with chronic liver cirrhosis being considered for major resection, preoperative portal vein embolization (PVE) should be considered as an option to induce hypertrophy in the future liver remnant, to minimize risk of postoperative liver failure ( Figure 7-2 ). Patients with HCC meeting the United Network for Organ Sharing (UNOS) criteria should be considered for liver transplantation (living or cadaveric). Situations that are beyond these criteria are controversial (e.g., resectable multifocal disease, patients with resectable tumors who are Child-Pugh Class A within the UNOS criteria) such that a multidisciplinary tumor board should be the platform for decision making.
Patients who are not candidates for potential cure or not medically suitable for major surgery, should be treated with loco-regional approaches. These are broadly classified into ablation techniques and transarterial embolization. Systemic chemotherapy should only be recommended to patients with unresectable HCC in a context of a clinical trial. There are modest but promising results in phases III trials using sorafenib (Nexavar) in both the Western and Asia Pacific regions when compared to best supportive care. , The details of loco-regional treatment and systemic treatment for advanced HCC are beyond the context of this chapter, and only surgical management will be discussed.
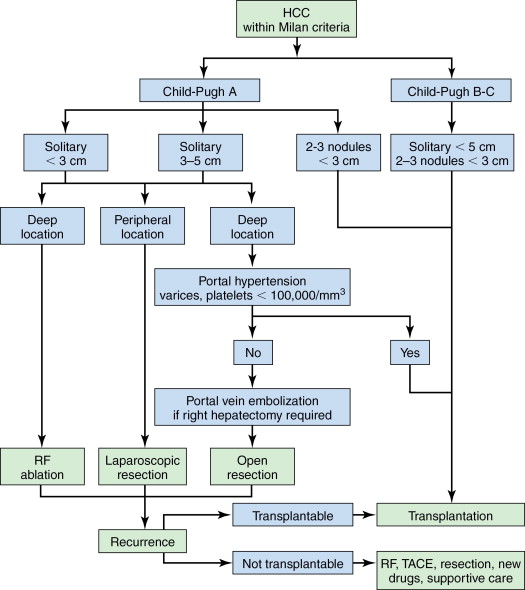
A general approach to treatment of HCC within Milan criteria is shown in Figure 7-3 . Our suggested approach is one example and is useful for conceptualizing the various treatment options that are available for individual patients but not negating the importance of a multidisciplinary HCC tumor board decision. Attempts to generate algorithmic approaches to the treatment of HCC are difficult because new treatments and indications for various treatments are evolving rapidly. A discussion of these issues and an overview of surgical therapies for HCC are summarized.
Comprehensive multidisciplinary care
Considering the complexity of the disease and the armamentarium of treatment options available, a multidisciplinary team should be the platform for all decision making in patients with HCC. The multidisciplinary HCC team should be inclusive of hepatobiliary/liver transplant surgeons, hepatologists/gastroenterologists, medical oncologists, dedicated liver radiologists, interventional radiologists and pathologists, and all relevant allied health care professionals. The team should meet regularly at a tumor board and liver transplant meeting with a review of the patients’ clinical status, radiologic images for surgical planning, and histopathology if available, after which an individualized treatment plan is decided by consensus. The meeting also provides an opportunity for the multidisciplinary HCC team to periodically review the latest relevant literature for an evidence-based management. Recommendations may be adapted to local regulations, team’s experience and ability as well as consideration of balancing cost and benefits.
Preoperative assessment
Patient assessment is key in the selection of candidates for liver resection or liver transplantation. As HCCs often present at a late stage, surgical resectability is a primary concern. Once it has been established that curative resection is indicated, resectability can be determined by various factors, including patient, disease, and technical factors. General patient factors include assessment of comorbidities for medical fitness for general anesthesia and major surgery. Specific patient and disease factors determined from two aspects: anatomic feasibility and volumetric tolerance. The main principle lies in the technical ability to leave a liver remnant with adequate function for sustaining life but consistent with low perioperative morbidity and mortality, acceptable long-term outcome in survival, and good quality of life. Relative contraindications to partial hepatectomy include significant medical comorbidities and/or extrahepatic disease. The golden rule for partial hepatectomy is to ensure enough liver parenchyma with a satisfactory blood supply (hepatic artery, portal vein, and hepatic vein), and biliary drainage remains after resection so that the patient does not develop postoperative liver failure. Contraindications of liver resection are situations in which the tumors have invaded the biliary confluence or invaded the three hepatic veins or the portal bifurcation. Any scenario in which bilioenteric anastomosis to the remaining bile ducts cannot be constructed is also considered as a relative contraindication to resection. A multidisciplinary team approach is recommended to validate the decision.
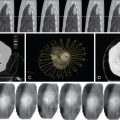
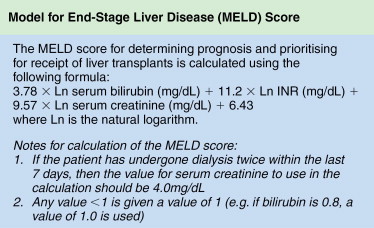
The ability of the nonpathologic liver to regenerate after liver resection is good and a remnant functional normal liver tissue as little as 20% to 25% may be sufficient. , The ratio of remnant functional liver to the initial total liver tissue may be difficult to estimate because of the size of the tumor or number of tumors (e.g., satellite nodules); the volume of these tumors should be excluded when determining the volume of the total liver. There are studies that utilize volumetric ratio without considering the total liver volume, but uses the percentage of total body weight instead. It has been shown that there is a risk of postoperative liver failure when the remaining functional liver ratio falls to less than 0.5% of total body weight. Computed tomography (CT) of the liver is usually applied to measure liver volumes. Most surgeons will not advocate resection if the estimated volume of functional remnant liver is either less than 20%–25% of the total liver volume or less than 0.5% of total body weight. In the West, many centers rely mainly on the Child-Pugh classification ( Table 7-1 ), but as a single assessment, it has significant variations and may underestimate the degree of cirrhosis. As a result, some centers include the clinical signs and radiologic evidence of portal hypertension to guide risk, and a study has shown that hepatic vein pressure of more than 10 mm Hg is a predictor of postoperative liver decompensation. Model for End-stage Liver Disease (MELD) is a prospectively developed and validated chronic liver disease severity scoring system that uses a patient’s laboratory values for serum bilirubin, serum creatinine, and the international normalized ratio for prothrombin time (INR) to predict survival ( Figure 7-4 ). It has been universally adopted by UNOS in 2002 and many parts of the world as a prioritizing tool for liver transplantation. Other than Child-Pugh scoring, MELD and liver function test, some studies have validated the use of indocyanine green clearance (ICG) as an objective adjunct in assessing liver function; in Asia, some institutions use ICG selectively for patient with tumors that are borderline resectable. In patients with “borderline resectability,” one safer option is to induce hypertrophy in the remaining functional liver via PVE to reduce the risk of postoperative liver failure. Compensatory hypertrophy of the contralateral lobe is generally observed in a period of 3–6 weeks (see Figure 7-2 ). Some studies report a high feasibility rate for this strategy and report a mean gain in liver volume of more than 40%, thus increasing the volumetric feasibility for liver resection.
| 1 Point | 2 Points | 3 Points | |
|---|---|---|---|
| Albumin (g/L) | >35 | 28–35 | <28 |
| Bilirubin (μmol/L) | <34 | 34–50 | >50 |
| INR | <1.7 | 1.71–2.20 | >2.20 |
| Ascites | None | Slight | Moderate |
| Encephalopathy | None | Grade 1–2 | Grade 3–4 |
| Points | Class | 1-y Survival | 2-y Survival |
| 5–6 | A | 100% | 85% |
| 7–9 | B | 81% | 57% |
| 10–15 | C | 45% | 35% |
Patients with significant comorbidities should be reviewed by the anesthetic team for evaluation of preoperative risk factors related to general health status (American Society of Anesthesiologists [ASA] score) or associated significant comorbidities to optimize preoperative status.
Surgery
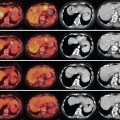
Curative liver resection is the optimal treatment for HCC in noncirrhotic patients and patients with Child-Pugh Class A cirrhosis. This is the treatment of choice for HCC in noncirrhotic patients, who account for about 5% of the cases in Western countries and for about 40% in Asia. A patient ideally suited for resection is one with a localized HCC confined to the liver without radiographic evidence of invasion of the hepatic vasculature, well-preserved hepatic function, and no evidence of portal hypertension ( Figure 7-5 ). , Notwithstanding aggressive surgical policies, the majority of patients have HCC or liver disease too extensive to permit resection with curative intent. In high-incidence regions of the world, only a minority of the patients are candidates for standard resection, about 10%–15% of newly diagnosed patients and, in low-incidence areas, between 15% and 30% of patients are potentially resectable.
Intraoperative staging
Intraoperative ultrasonography (IOUS) may improve the selection of patients for potentially curative resection. Intraoperative ultrasound can accurately determine the size of the HCC and detect portal or hepatic vein involvement, which precludes resection. In a study that included 91 patients with HCC who underwent IOUS prior to resection, and no surgery was performed in 16% because of unresectable disease. Another advantage of IOUS to the surgeons is the identification of major intrahepatic vascular structures, which can be used to guide nonanatomic or segmental resections. Intraoperative ultrasound can be done before resection in open surgery or via a laparoscopic technique.
Surgical technique
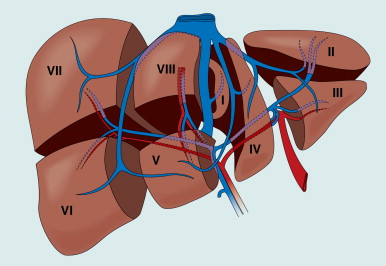
Hepatocellular carcinoma has a predilection for invading the portal vein, leading to intrahepatic metastasis, providing the basis for the preference for anatomic resection, along with the principles of surgical oncology. In the noncirrhotic liver, an anatomical resection should be performed, following Couinaud segments ( Figure 7-6 ). Anatomic liver resection is achieved by selective ligation of the inflow portal triad and ligation of dominant outflow hepatic veins to the segments or segment resected. If subsegmental resection is planned, IOUS enables precise identification of the intrahepatic vessels during segmentectomy. For cirrhotic patients, the technical approach can be modified, with resection encompassing the least amount of nonmalignant parenchyma feasible, so as to maintain satisfactory postoperative liver function. Importantly, both anatomic and wedge resection are acceptable as long as there are adequate clear margins of at least 1 cm. Anatomic resections aiming at 2-cm margins provide better survival outcome than narrow resection margins less than 1 cm. The potential for liver regeneration is impaired in cirrhotic patients; thus, resection is generally more conservative. However, some patients maintain adequate functional liver reserve even after a formal hemihepatectomy, particularly if preoperative PVE is used to induce compensatory hypertrophy in the future liver remnant.
Surgical techniques have improved over the past decade as a result of advances in surgical techniques and better experience. These include equipment for parenchymal transection, for example, ultrasonic dissector (CUSA, Tyco Healthcare, Mansfield, MA) and the Hydrojet (Hydro-Jet, Erbe, Tubingen, Germany) and various surgical staplers for pedicle or vascular transections. Operative maneuvers include vascular occlusion techniques like the intermittent use of the Pringle maneuver for vascular inflow occlusion as an alternative to total vascular occlusion (clamping of the portal vein, hepatic artery, and the inferior vena cava [IVC]). Intermittent Pringle occlusion (e.g., 15 to 20 minutes of clamping with 5 to 10 minutes of unclamping) is well tolerated by cirrhotic patients for up to a total of an hour and is better tolerated than continuous clamping. , Intraoperative anesthetic management (e.g., low central venous pressure maintenance) and immediate postoperative care have been optimized. These strategies have led to a decrease in blood transfusion from about 80% to less than 10% in two decades. , The conventional approach to resection of large right-sided HCC consisted of complete mobilization of the right liver followed by vascular control and then tumor resection.
Some surgeons have advocated a “no touch” technique or the anterior approach to resection of HCC. This approach utilizes initial anterior parenchymal transection of the liver parenchyma down to the IVC, and ligation of the inflow and outflow vessels before mobilization of the liver. The advocates hypothesize that the separation of the right liver and the tumor from the IVC before mobilization avoids prolonged rotation and displacement of the liver and manipulation of the tumor, thus reducing the risk of vascular rupture. In addition, division of the vessels before tumor manipulation theoretically minimizes the potential for tumor cell dissemination caused by tumor compression. , The liver hanging maneuver described by Belghiti describes the elevation of the posterior aspect of the liver by gently lifting the liver with a surgical tape passed between the anterior surface of the IVC and the liver parenchyma. This liver hanging maneuver during transection can be useful for improving exposure, especially when addressing large, right-sided tumors involving the diaphragm ( Figure 7-7 ).
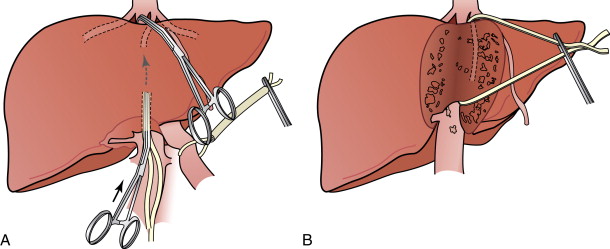
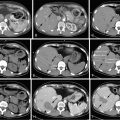
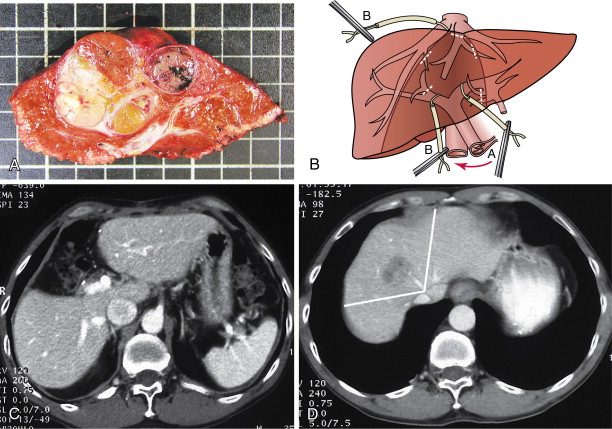
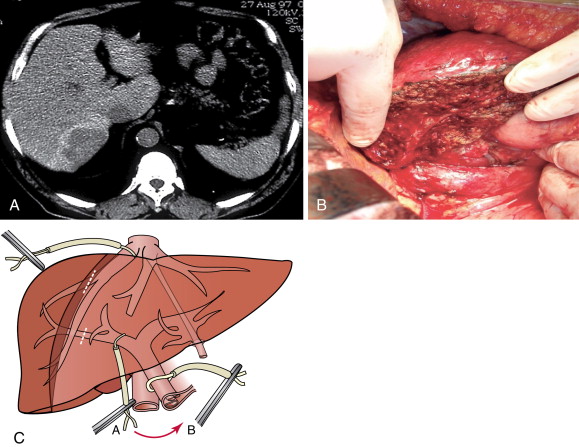
Centrally located HCC (segments 4, 5, and 8) can be technically more challenging ( Figure 7-8 ). Extended hemihepatectomy is the treatment of choice if potentially curative surgery can be undertaken safely. However, this conventional approach can be associated with high perioperative morbidity and mortality rates due to inadequate residual functional liver parenchyma. An alternative segment-oriented approach, central hepatectomy, has been proposed in which the central liver segments 4 and/or 5, and 8 (with or without segment 1) are removed and the lateral sectors remain intact. Current literature indicate that the available data suggest that central hepatectomy (mesohepatectomy) is an alternative to extended hemihepatectomy for centrally located tumors, providing acceptable oncologic outcomes with less liver parenchymal loss. However, it is a complex and technically demanding procedure that requires two hepatic resection planes and bilateral biliary reconstruction. Consequently, this translates to a higher risk of bile leak and bleeding as well as long-term biliary stricture and biliary dysfunction. Along the same principles, anatomic bi- or trisegmentectomy is also a safe alternative to extensive liver resection in selected patients with selective vascular occlusion ( Figure 7-9 ).
Laparoscopic liver resection
The success of minimally invasive surgery and experience in resection of benign hepatic tumors along with advances in surgical technology has driven interest in laparoscopic approaches to surgery for HCC. , Development of advanced energy devices for parenchymal transection, for example, harmonic scalpel (Ultracision; Ethicon), which is a thermofusion device (Ligasure; Tyco Healthcare) also assisted in the advancement of laparoscopic liver resection. Indications and contraindications for laparoscopic liver resection are also well defined ( Table 7-2 , see Figure 7-6 ). Liver resections, including major hepatectomies, are now performed by laparoscopy in specialized centers. , Preliminary results in the literature suggest that laparoscopic liver resections are associated with fewer postoperative general complications, less intraoperative bleeding, less postoperative analgesic demand, and reduced hospital stay when compared to open procedures. Some series, with small numbers of patients undergoing liver resection for HCC, show that the benefit of laparoscopic over open procedures seems to be greater than in other indications of liver resection. Especially in cirrhotic patients, there is less liver mobilization and the amount of intraoperative intravenous fluid is reduced. Because of the minimal insensible fluid loss as compared to an open procedure, the third space fluid accumulation is reduced and this resulted in fewer postoperative ascites. Moreover, as a result of the significantly smaller wounds, abdominal wall complications were also decreased by the use of laparoscopy. , Most importantly, patient safety and oncologic outcomes must be of highest priority in the consideration of laparoscopic liver surgery. It is also highly technically demanding and should be undertaken only in high-volume centers with a program of graduated procedural complexity.
| INDICATIONS |
| Location: Segments 2 through 6 |
| Pathology |
| Benign and malignant neoplasms |
| Cystic lesions |
| Parasitic lesions |
| Size |
| Lesions <5 cm |
| Left lateral section lesions of any size not in proximity to major vascular |
| structures |
| Pedunculated neoplasms of any size not in proximity to major vascular structures |
| RELATIVE CONTRAINDICATIONS |
| Location |
| Lesions near the inferior vena cava, hilum, or distal hepatic veins |
| Multiple or bilateral lesions |
| Segments 1, 4a, 7, or 8 |
| Pathology |
| Coagulopathy or thrombocytopenia |
| Moderate to severe portal hypertension |
| Previous operation, especially upper abdominal |
| Size |
| Laparoscopic approach requiring a larger parenchymal resection than would be necessary via an open approach |
| Nonpedunculated benign and malignant neoplasms >5 cm |
| ABSOLUTE CONTRAINDICATIONS |
| Gallbladder cancer and hilar cholangiocarcinoma |
| Inability to obtain a surgical margin that would otherwise be possible in an open resection |
| Patient unable to tolerate an open operation or pneumoperitoneum |
| Insufficient surgeon training |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree