Step 1
Surgical Anatomy
- ♦
The larynx is composed primarily of the upper thyroid cartilage and the lower ringlike cricoid cartilage.
- ▴
The thyroid cartilage is shield-shaped with a left and a right lamina that rests on the lower cricoid cartilage through the cartilaginous cricothyroid joint posteriorly. The vocal cords are located approximately halfway down the thyroid cartilage, projecting as two folds of epithelium-covered muscle in the laryngeal lumen. The midline notch in the thyroid cartilage represents the Adam’s apple.
- ▴
The two arytenoid cartilages, right and left, sit on the back portion of the cricoid (the cricoid posterior lamina) and are the posterior attachments of the vocal cords, which anteriorly insert into the inner surface of the midline thyroid cartilage. The arytenoid cartilages have a variety of motions that are possible through their cricoarytenoid joints. The basic motion of the arytenoid cartilages is rotation, either medially or laterally. When rotating laterally, they cause abduction of the vocal cord; when rotating medially, they cause adduction of the vocal cord. Figure 6-1 shows the thyroid and cricoid cartilages of the larynx and the upper cervical trachea with the left thyroid lamina removed.

Figure 6-1
- ▴
- ♦
The laryngeal musculature can be divided into those muscles that result in vocal cord adduction and those that cause vocal cord abduction.
- ▴
Adductors of the larynx include the thyroarytenoid muscle (also known as the vocalis muscle ), the lateral cricoarytenoid muscle, and the interarytenoid muscle. The thyroarytenoid muscle represents the muscular substance of the vocal cord.
- ▴
The main abductor of the vocal cord is the posterior cricoarytenoid muscle, which is located on the dorsal surface of the posterior lamina of the cricoid.
- ▴
- ♦
The cricothyroid muscle is the only laryngeal muscle present within the thyroid surgical field; it occurs on the anterolateral face of the larynx at the lower margin of the thyroid cartilage and the upper margin of the anterior ring of the cricoid cartilage. In this position the cricothyroid muscle, when active, acts to tilt the thyroid cartilage forward on the underlying cricoid cartilage and in this way tenses the vocal cord ( Fig. 6-2 ).
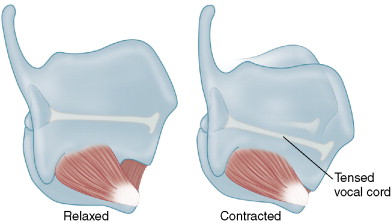
Figure 6-2
- ♦
The recurrent laryngeal nerve (RLN) carries branchial efferents to the inferior constrictor muscle of the pharynx, cricopharyngeus muscle, and all intrinsic laryngeal muscles except for the cricothyroid muscle. General visceral afferents from the larynx (at the level of the vocal cords and below), upper esophagus, and trachea are also carried within the RLN. The RLN also carries sympathetic and parasympathetic innervation to the lower pharynx, larynx, trachea, and upper esophagus. Laryngeal cortical representation projects to bilateral brainstem nuclei, primarily the nucleus ambiguus, which in turn projects to the ipsilateral larynx musculature.
- ♦
The mean diameter of the RLN ranges from 1 to 3 mm. The vagal to larynx length on the left averages approximately 14 cm and on the right approximately 12 cm. The RLN in its proximal course is nearly half motor fibers and half sensory fibers. The bulk of sensory fibers are given off before laryngeal entry, with nearly 80% of fibers entering the larynx being motor fibers.
- ♦
The adductor fibers (fibers leading to the adductor laryngeal muscles) outnumber abductor fibers within the RLN by 3:1 or 4:1. It is generally thought that there is no spatial segregation of abductor and adductor fibers within the RLN.
- ♦
The superior laryngeal nerve (SLN) is one of the first branches of the vagus nerve, usually separating from the vagus nerve at the nodose ganglion, approximately 4 cm cranial to the carotid artery bifurcation. The SLN divides into an internal branch, which provides afferent supply for the supraglottic larynx, entering the larynx laterally in the thyrohyoid membrane. The external branch of the SLN extends further inferiorly and provides branchial efferents to the cricothyroid muscle. The nerve continues through the two heads of the cricothyroid muscle to innervate the anterior third of the vocal cord.
- ♦
Basic laryngeal movement is divided into adduction, which is the movement of the vocal cords to the midline, and abduction, which is the lateral movement of the vocal cords away from the midline. As one inspires, abductor function predominates, allowing lateral cordal motion and widening of the glottis. During phonation, adduction tone predominates.
- ♦
Voice represents the passage of air exhaled through the partially closed and tensing vocal cords. The movement of this exhale sets up an epithelial vocal cord cover oscillation known as the cord’s mucosal wave . Voice intonation results in subtle variations in muscular tension during this process. Higher registers through vocal cord tension are also controlled through cricothyroid muscle contraction.
- ♦
The epithelial wave that characterizes the human voice can be best seen on stroboscopic evaluation of the larynx. Any subtle disruption or irregularity of the luminal surface of the vocal cord results in a disruption of this process and a change to the voice that is termed hoarseness . The characteristic change that occurs from poor vocal cord apposition during phonation, as occurs with vocal cord paralysis, is more a breathy quality to the voice rather than true hoarseness. The voice of vocal cord paralysis is thus felt to be underpowered and characterized by a breathy, weak voice with poor projection (see Video 6-1: Laryngoscopy: Normal and abnormal findings).
Step 2
Preoperative Considerations
- ♦
Preoperative laryngeal examination is tremendously helpful to the endocrine surgeon because we cannot rely on the patient’s vocal symptoms to predict glottis function. There is a well-known divergence between vocal symptoms and glottic function. Thus, patients who are noted to be hoarse may not have vocal cord paralysis and patients with vocal cord paralysis present preoperatively may not be hoarse.
- ♦
Symptomatic assessment is unreliable due in part to variations in vocal cord position with vocal cord paralysis as well as the potential for contralateral vocal cord compensation. Preoperative vocal cord paralysis may occur in the setting of malignant infiltration of the nerve, from prior thyroid or neck-based surgery, but may also occur in the setting of benign disease. In our series overall the rate of preoperative vocal cord paralysis in patients who had not undergone prior surgery was 4%. Several series suggest that up to 50% to 60% of patients presenting with preoperative vocal cord paralysis may be asymptomatic. This is not surprising because of the prolonged time frame of malignant infiltration of the nerve and the tendency for contralateral cord compensation.
- ♦
We recommend consideration for laryngeal examination in all patients undergoing thyroidectomy. Preoperative laryngoscopy is a readily available diagnostic tool. Identification of preoperative vocal cord paralysis basically makes the diagnosis of extrathyroid disease evident preoperatively and allows the surgeon to better radiographically evaluate the patient and more intelligently plan the surgery, which may involve tracheal or laryngeal surgery, and allows for better preoperative patient counseling.
- ♦
In our series we found that 70% of patients with invasive disease have preoperative vocal cord paralysis. The majority of these patients did not have hoarseness as a preoperative symptom. In short, preoperative vocal cord paralysis tracks reliably with invasive thyroid disease, which is of significant importance in surgical planning. The surgeon cannot rely on voice or radiographic evaluation to detect vocal cord paralysis.
- ♦
Glottic examination with preoperative laryngoscopy is essential. It is of note that management of the nerve found invaded at surgery depends in part on the preoperative functional status of the vocal cord. It is therefore essential for rational operative management to be aware of the preoperative functional status of the cord.
Step 3
Operative Steps
Vagus Nerve and RLN Surgical Anatomy
- ♦
The right vagus nerve, traveling ventral to the right subclavian artery, gives off the RLN in the thoracic inlet. The right RLN then travels up the paratracheal region from lateral to medial as it ascends the neck. It typically holds a more oblique course relative to the side of the trachea than the left RLN, which travels in a strictly cranial direction. On the left, the vagus nerve travels ventral to the aortic arch. The left RLN derives as an upward-going branch of the vagus nerve and ascends the neck, typically in the tracheoesophageal groove in the left paratracheal region.
- ♦
As the RLN ascends the neck, the first meaningful anatomic event is typically the crossing of the RLN with the inferior thyroid artery. This has been regarded as a stable landmark for RLN identification. However, the RLN and inferior thyroid artery enjoy quite a bit of variability in their cervical courses. There are nearly 30 different patterns of crossing of nerve to artery.
- ♦
In addition, a substantial percentage of patients have a varying relationship of RLN to inferior thyroid artery from one side to another. It is of note that up to 8% of patients may not have an RLN–inferior thyroid artery crossing point. Because of this variability, this RLN–inferior thyroid artery crossing is not the ideal landmark that it has been purported to represent.
- ♦
As the nerve travels superiorly above the arterial crossing point, it extends up toward the ligament of Berry. As it does this, it travels in close proximity with the lateral aspect of the thyroid midpole region.
- ♦
In this area, a variety of nodular formations on the lateral and posterior surface of the thyroid may interact with the nerve in its distal course. The most notable of these nodular formations is felt to be a stable, posteriorly directed nodule that extends laterally and posteriorly and is termed the tubercle of Zuckerkandl . Although this has been described as a stable finding, it has been our experience that nodularity of the midaspect and posterior aspect of the thyroid lobe is quite variable and would represent an inconstant anatomic landmark for nerve identification.
- ♦
It is of note that because of the variability in nodular formation in this area, the nerve must be followed carefully throughout its distal course, which can have a variable relationship to the thyroid’s lateral and posterior edge.
- ♦
As the nerve extends more superiorly, it relates to the ligament of Berry, which is a bilateral structure that represents the thyroid’s attachment to the upper airway; this has been described beautifully by both Drs. Berry and Berlin (see References). Typically the nerve is dorsal and posterior to the insertion of the ligament of Berry on the upper cervical trachea and lower cricoid.
- ♦
In certain patients, however, the ligament of Berry may have both anterior and posterior leaflets and the RLN may travel through or between these leaflets. In this way a portion of the ligament of Berry may extend dorsally, posterior to the nerve. This implies that retraction of the thyroid is conveyed through the ligament of Berry to the nerve. This can result in an upward bowing of the nerve, which may result in, at the very least, neurapraxic stretch injury in certain circumstances. We feel this may occur even with appropriate thyroid lobe retraction and is likely to be the most frequent mechanism of injury of the RLN during thyroidectomy.
- ♦
The ligament of Berry may be considered a condensation of the true thyroid capsule, so it may be infiltrated with thyroid tissue. This represents one of the three areas of the thyroid that can be left behind during total thyroidectomy ( Fig. 6-3 ). The variable degree of infiltration of thyroid tissue within the ligament of Berry can bring thyroid tissue into very close approximation to the nerve. In some circumstances, thyroid tissue may in fact come to rest posterior to the nerve at its laryngeal insertion point.
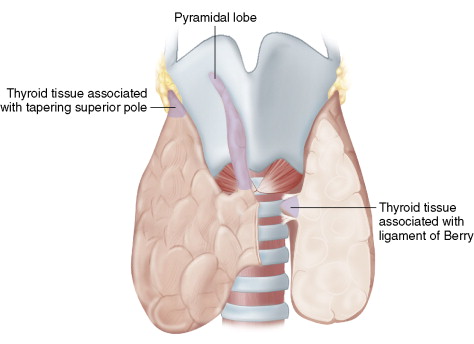
Figure 6-3
- ♦
The degree of dissection of the RLN and decisions about how much tissue, if any, to leave at this difficult and key region containing the ligament of Berry depend on how robust the nerve is expected to be to surgical dissection. If the nerve is of a wide caliber, of round profile, and could tolerate dissection, we endeavor to excise all thyroid tissue in this area. However, if the nerve is thin, flat, and of small caliber (as is often seen in tall, thin women), aggressive dissection in this area will increase the risk of neurapraxic injury. In these circumstances we consider leaving a small remnant of thyroid tissue at the ligament of Berry. It is important to leave tissue that is anodular and away from any known thyroid cancer.
- ♦
The ligament of Berry is a difficult area to work on in that the fascia can be thickened, tough, and well-vascularized, being provided a separate branch of the inferior thyroid artery. It is essential to work slowly and meticulously in this area. If bleeding is engendered, slight pressure with digital application of gauze is recommended because aggressive pressure with instrument or injudicious liberal cautery may result in RLN injury. Bleeding in this area, although it may obscure the nerve, is typically from a very small diameter of vessel and so simple digital pressure with a gauze pad for a moment or two often will allow the surgeon to identify the troublesome small vessel and apply a clip, tie, or judicious fine-tipped bipolar cautery. Because of the variation in relationship of the nerve to thyroid tissue, depending on the degree to which thyroid tissue infiltrates the ligament of Berry, it is essential to follow the nerve through the ligament of Berry rather than strictly following the thyroid capsule in this area.
- ♦
The RLN, as it travels from the inferior aspect of the thoracic inlet to the undersurface of the cricoid cartilage to enter the larynx, gives off multiple branches. The majority of these branches are sensory ones to the trachea, esophagus, hypopharynx, and larynx.
- ♦
Extralaryngeal motor fiber branching refers to a premature division of laryngeal motor fibers before the laryngeal entry point (defined as the entry of the RLN under the inferiormost edge of the inferior constrictor muscle as it enters the larynx). Through the use of intraoperative monitoring, we determine that such premature extralaryngeal motor fiber branching occurs in approximately 10% of patients; we consider these branches as premature intralaryngeal branches seen below the lowermost fibers of the inferior constrictor. Nearly 90% of such extralaryngeal motor fiber branching that can be visualized in the thyroidectomy surgical field occurs in the distal course of the nerve, above the nerve crossing point with the inferior thyroid artery.
- ♦
Studies show that such motor fiber branching patterns may vary from side to side. With neural monitoring, one can deduce what is a true motor fiber and what is a sensory fiber. This is difficult, if not impossible, to determine through visual inspection alone.
- ♦
When true extralaryngeal motor fiber branching is present, the anterior branch will contain fibers destined for adductor musculature. The posteriormost branch is primarily sensory, being the RLN’s contribution to a robust posterior laryngeal sensory anastomosis between the RLN from below and the SLN from above, termed the nerve of Galen . A variety of studies and our clinical experience suggest abductor fibers (i.e., fibers of the RLN destined for the posterior cricoarytenoid muscle) may be present in the anterior branch or in the posterior branch. Given this lack of predictability as to motor fiber location, all branches of the RLN should be preserved.
- ♦
The RLN can give important information regarding parathyroid localization and identification. If the RLN is taken as a coronal plane in the neck, the inferior parathyroid is reliably found ventral (i.e., superficial) to this plane, whereas the superior parathyroid is reliably posterior or dorsal to this plane.
- ♦
The position of the parathyroid relative to this coronal plane, in our experience, is more reliable in terms of differentiating inferior from superior gland, than the parathyroid gland’s position along the cranial-caudal axis. Parathyroid glands may be juxtaposed in quite close approximation along a cranial-caudal axis, yet can be reliably differentiated based on their relative position to the coronal RLN plane in the neck. The superior parathyroid often will be just posterior and deep to the RLN, just prior to the RLN entry point into the larynx ( Fig. 6-4 ).
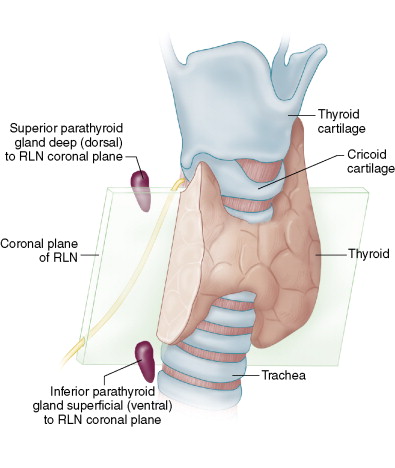
Figure 6-4
- ♦
The entire course of the RLN in the neck is demonstrated in Figure 6-1 . Of note, during the RLN’s distal course, it relates to the ligament of Berry, either traveling behind or through the ligament of Berry, and then at the lowest edge of the lateral cricoid cartilage enters the larynx, dipping beneath the lowermost fibers of the inferior constrictor muscle of the larynx ( Fig. 6-5 ). We term this point the laryngeal entry point —the point at which the RLN leaves the thyroidectomy surgical field. It is of note that in cases of malignant infiltration, where dissection of an additional segment of nerve is necessary or helpful, the lowermost fibers of the inferior constrictor muscle can be divided and one can identify a 5- to 7-mm section of additional nerve that can be useful in certain cases of invasive disease involving the distal RLN course.
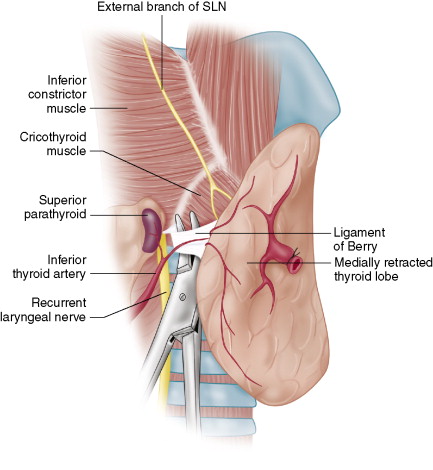
Figure 6-5
Surgical Approaches to the RLN
- ♦
The surgical approach to the RLN may vary depending on the type of case. The approach to the nerve can be empirically divided into three specific approaches. Keep in mind that a nerve identified through any technique is a nerve well identified. Nonetheless, three separate perspectives on nerve identification can be helpful during thyroidectomy.
The Inferior Approach to the RLN
- ♦
In this approach, the RLN is identified at the thoracic inlet laterally for the right RLN and typically more medially in the tracheoesophageal groove on the left. The RLN, in this inferior approach, is found in a loose areolar bed of the lower paratracheal region and here, proximal to the inferior thyroid artery crossing point, typically exists as a single trunk prior to any extralaryngeal nerve branching. This can be helpful in the setting of revision surgery, where the nerve can be found in an undissected region caudal to the previous surgery scar. The downside of this approach is that it involves dissection of a long segment of the RLN. One must be cautious that, in this long cranial-caudal length of RLN dissection, the inferior parathyroid gland is not devascularized. One must also consider in this approach on the right the potential for a nonrecurrent right RLN ( Fig. 6-6A ).
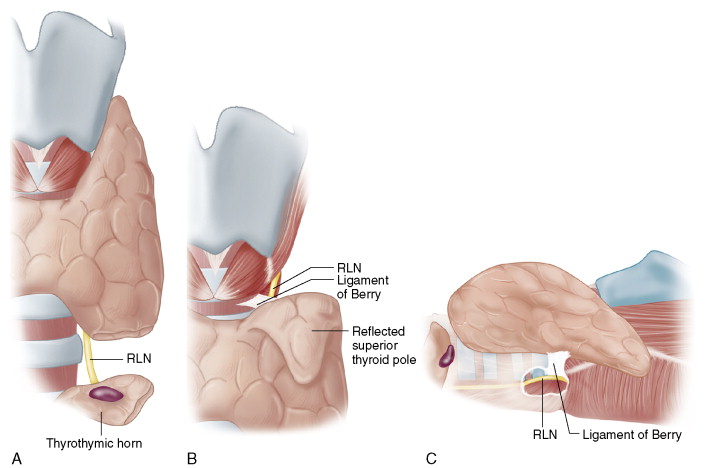
Figure 6-6
The Superior Approach to the RLN
- ♦
In the superior approach the RLN is found at the laryngeal entry point just above the ligament of Berry. The advantage of this approach is that the RLN’s entry to the larynx represents its most constant position in the neck. The larynx may be deviated or rotated, but the nerve will always relate to the lateral cricoid and enter at the cricoid’s inferior edge.
- ♦
This approach is especially useful for large cervical or substernal goiters, where the nerve cannot be found inferiorly or laterally. The inferior cornu of the thyroid cartilage may be palpated to approximate the location of the nerve in this region.
- ♦
The nerve is also more easily located in this area once one is oriented to the anterior arch of the cricoid anteriorly. Once this region is identified, nerve stimulation can be helpful to focus the dissection. Once the nerve is identified in this area, it can be dissected in a retrograde fashion and reflected off the undersurface of the thyroid lobe.
- ♦
The disadvantage of this approach is that the ligament of Berry is fibrous and well vascularized. The superior pole also must be taken down first so the external branch and superior parathyroid must first be identified and avoided. This approach may be more technically challenging with a large superior pole. However, this is a very useful and reliable method for nerve identification when other approaches fail or in the setting of a nonrecurrent RLN ( Fig. 6-6B ).
The Lateral Approach to the RLN
- ♦
In the most commonly used approach, the lateral approach, the RLN is found laterally at the midpole level with medial thyroid lobe retraction. This is applicable to routine cases. The advantage is that it protects the parathyroid vascular supply and limits the length of RLN dissection. It does, however, dissect the RLN over the region where thyroid tissue is brought close to the nerve, just at and below the ligament of Berry.
- ♦
This is not an available approach for large cervical or substernal goiters or if there is extensive scar from previous surgery. It is also of note that because the nerve is identified quite distally in its course, extralaryngeal branching may occur at this level; caution must be applied as one approaches a very small-caliber nerve laterally. This approach is ideal in the majority of first- time nonrevision operations for a nongoiterous gland ( Fig. 6-6C ).
Vagus Nerve and RLN Intraoperative Neural Electrophysiologic Monitoring
- ♦
Recurrent laryngeal nerve monitoring can be divided into two modes of application: passive monitoring and evoked monitoring.
- ▴
Passive monitoring is a neural monitoring mode in which passive activity of the RLN is identified through the surface electrodes that are monitoring the bilateral vocal cords.
- ▴
In evoked monitoring, the nerve is provocatively stimulated with a sterile stimulation probe, and the resulting depolarization of the RLN and ipsilateral vocal cord is measured. This probe stimulation facilitates neural identification because non-neural tissue stimulation results in no electromyographic (EMG) activity and neural stimulation results in EMG activity.
- ▴
- ♦
A variety of equipment options are available for neural monitoring. We favor an endotracheal tube–based system, which allows for ongoing real-time EMG waveform data to be visualized on the monitor, giving quantifiable amplitude and latency information, as opposed to more simplified audio-based systems in which EMG activity simply results in an audio signal response. The information contained within the evoked waveform (i.e., amplitude, latency, waveform morphology) can be very informative.
- ♦
Neural monitoring results in electrical information regarding the nerve. This is best considered as an adjunct to visual information regarding the nerve.
- ♦
The nerve is always revealed at surgery in visual increments. Initially, it is identified as a slight, white region in the dissection field. Additional dissection allows one to recognize the lateral and medial margins of the nerve. Visual information as the nerve is dissected results ultimately in the identification of the trajectory of the nerve, cranial to caudal and, especially on the right, lateral to medial. Additional dissection of the nerve often allows one to identify the characteristic serpentine redundancy characteristic of linear neural structures and also the characteristic vasonervorum, or racing stripe, on the ventral surface of the nerve.
- ♦
All of these visual features are revealed in small and additive increments. Electrical stimulation and electric neural confirmation add to the progressively obtained visual information. We have found that more information (i.e., electrical and visual) in good surgical hands is very helpful.
- ♦
We have found this especially true in cases in which the visual information is problematic. This may occur, for example, in a woman with a long, thin neck in whom the nerve is of very reduced caliber and flat, in distinction to the thicker-caliber, rounded nerve that may be present in a larger male. The visual information from such a small-caliber nerve can be augmented by electrical stimulation. Neural stimulation can also be helpful as the nerve is dissected through a difficult ligament of Berry, because ongoing electrical stimulation with adequate amplitude implies ongoing neural health without neurapraxia.
- ♦
In some circumstances the nerve can be tented up at the ligament of Berry or bowstringed by the distal inferior thyroid artery as the lobe is retracted in the final portions of the dissection. Induced stretch neurapraxia cannot be detected visually but can be detected electrically and can result in re-evaluation of the surgical maneuver that induced the change in electrical stimulability. This is information that is not available visually.
- ♦
Electrical stimulability also allows the surgeon to identify all motor fibers entering into the larynx and allows the surgeon to appreciate which are motor and which are sensory branches of the nerve. This is also information that is not apparent through even loupe-magnified visual evaluation.
- ♦
Because neural monitoring represents a new technique, it has been the subject of great controversy. Intraoperative neural monitoring has been evaluated by many through the limited lens of studies looking at the rates of paralysis with or without monitoring. The vast majority of these studies are insufficiently powered and deny the many categories of benefit that typically become apparent to a surgeon through routine application of neural monitoring after only several months. Currently, it is estimated that approximately 40% of U.S. thyroid surgery is monitored.
- ♦
Neural monitoring does not in any way replace the need for surgical skill or the need for detailed anatomic knowledge. However, it does represent a new functional dynamic during surgery that was not previously available. Neural monitoring really represents additional information available to the surgeon at surgery. It does not replace the need for visual identification of the nerve and requires a strictly bloodless field. There is a learning curve and a period of adjustment for the intricacies of neural monitoring to be mastered.
- ♦
Although recent data suggest that neural monitoring is accurate in prognosticating postoperative neural function, at the present time we feel that laryngeal examination is absolutely essential and wedded to neural monitoring, both preoperative and postoperative, in all cases.
- ♦
We have found neural monitoring in its evoked mode (i.e., through nerve stimulation) to be very sensitive. Stimulation as close as 1 mm next to the nerve will result in no nerve response, whereas stimulation on the nerve will result in a positive response.
- ♦
When the nerve is stimulated, it is important that there is not excessive obscuring overlying fascia and that the field is bloodless. Just as cerebrospinal fluid will shunt away current when the facial nerve is stimulated at the cerebellopontine angle, so will tissue, fluid, or blood in the neck base overlying the nerve shunt current away from the nerve and give a false-negative response.
- ♦
We have studied the monitoring endotracheal tube surface electrode arrays in comparison to the thyroarytenoid intramuscular EMG hook wire electrode, considered the standard laryngeal EMG assessment, and found that the endotracheal tube surface electrode arrays provide equivalent and accurate sensitive laryngeal EMG measurement. We feel that the endotracheal tube surface noninvasive arrays are an excellent method for intraoperative vocal cord monitoring, and that invasive needle intralaryngeal electrodes, or electrodes placed through the cricothyroid membrane into the cord, are unnecessary.
- ♦
It is important to understand that there is a dose-response relationship between electrical stimulation and EMG response and that the nerve begins to stimulate in a bloodless field with overlying fascia dissected free at 0.3 to 0.4 mA. As the stimulation current to the nerve is increased, the EMG amplitude increases to a maximum of approximately 0.8 mA. Again, this maximum is reached in the setting of a bloodless field with overlying fascia dissected. At this point further increases in stimulation current do not result in further increases in EMG amplitude (i.e., EMG threshold has been reached). Given this we recommend that the bulk of intraoperative neural stimulation be performed at the 1-mA current level. We have stimulated at this level in patients during experimental protocols hundreds of times and have not witnessed any case of stimulation-induced neural injury or neurapraxia.
- ♦
If one increases the stimulation current to 2 mA, there is no further increase in threshold EMG activity, but there is an increase in the current spread beyond the stimulation probe to a wider- diameter sphere around the tip of the probe. This has utility when searching for the nerve. Therefore, when searching for the nerve we use the stimulation probe at 2 mA. Once the nerve is identified visually and dissected, the stimulation is turned down to 1 mA for the remainder of the case. As noted above, when stimulated at 1 to 2 mA we have not witnessed neurapraxic injury, bradycardia, or bronchospasm in any patient through vagal and RLN stimulation with nearly 1400 nerves at risk.
- ♦
With bilateral electrodes one can frequently identify a contralateral waveform (i.e., waveform of the vocal cord contralateral to the nerve that is being stimulated). This simply represents the contralateral cord’s witnessing of the ipsilateral vocal cord’s depolarization.
- ♦
Monitoring can be helpful in three main modes: for identification, as an aid in dissection, and in prognostication of postoperative function.
- ▴
Neural monitoring as an adjunct for nerve identification has great utility. It does not replace the need for visualization of the nerve, but adds to the certainty of nerve identification, as the nerve is progressively visually uncovered. Neural mapping is the procedure whereby the nerve, undissected and unseen in an undissected region, is mapped out with neural stimulation and a likely trajectory of the nerve through the region is identified. This allows for rational and efficient directed dissection to then visualize and uncover the nerve.
- ▴
Neural mapping involves the identification of the paratracheal region after the thyroid lobe has been preliminarily dissected with the middle thyroid vein taken. Clear-cut identification of the midline trachea is necessary once the lobe is preliminarily dissected. In the undissected paratracheal region, a nerve stimulator can be used to trace out the projected course of the nerve through the paratracheal region at the level of the inferior pole. The stimulator probe can be used on 2 mA for this purpose, as noted above. Once a line of stimulation that results in positive neural depolarization is identified, stimulation just lateral and medial to this line will result in no neural stimulation. With this information the potential path of the nerve through this region is identified; then dissection can be targeted, leading to efficient nerve identification with a minimum of dissection. As has been discussed, as the nerve is identified, stimulation of the nerve can help to confirm its identity and differentiate nerve from blood vessel, allowing for segregation of motor fibers from sensory fibers.
- ▴
- ♦
Neural stimulation can be used as an aid in dissection once the nerve is visualized as it is being followed through the surgical field. This is similar to the use of intermittent facial nerve stimulation during parotidectomy. After the facial nerve has been identified, bands of tissue above the nerve are stimulated as negative, the nerve is stimulated as positive, and then the overlying band of tissue is taken. The dissection of the RLN, especially through difficult fields of dissection, such as the ligament of Berry, or difficult scarred revision fields, can be aided with intermittent stimulation. Neural tissue and small branches of the nerve give positive stimulation; adjacent fibrous tissues do not. The negative stimulation of adjacent fibrous tissue in the setting of positive neural stimulation allows for division of the non-neural tissue with impunity.
- ▴
Neural monitoring in its passive mode also can be helpful in that intermittent trauma-induced depolarization of the nerve implies nerve stretch or some type of nerve trauma. When identified, such depolarizations can lead to re-evaluation of the current surgical maneuver.
- ▴
Nerve stimulation is also an aid to dissection when training residents and fellows. Because the nerve is dissected through a field, the trainee can reconfirm nerve identification and location and confirm the non-neural nature of the tissue that is intended to be divided. We have found that the inclusion of neural monitoring in the operating room is well accepted by trainees, who typically use the nerve stimulator throughout the entire case, understanding that their surgical detection is being held to a higher standard (i.e., ongoing normal nerve function that can be intermittently checked). This intermittent ability to check the functioning of the nerve can allow for more complete nerve dissection, for example, at the ligament of Berry, with more complete thyroid tissue resection.
- ▴
Probably the most interesting mode of neural monitoring, which has the greatest utility, is in prognostication of nerve function at the completion of the initial ipsilateral side of a planned bilateral procedure. Such stimulation at the end of lobectomy at the level of the vagus nerve allows complete testing of the nerve circuit on this side and allows the surgeon to plan the second side with the first side’s function in mind. Such an algorithm should make the likelihood of bilateral cord paralysis and tracheotomy of interest only in the historical annals of thyroidectomy. Currently the only information that we have in the limited literature on this subject and from more recent quality registers implies that the only current test of neural function at the end of lobectomy, that being visual inspection of the nerve, allows only 10% of neural injuries that occur to be detected. Thus, with visual examination at the end of lobectomy, only 1 out of 10 nerve injuries is appreciated. This is in distinction to EMG signal stimulation at the level of the vagus nerve at the conclusion of lobectomy, which in our series has a negative predictor value of 99.6%.
- ▴
- ♦
Should the EMG signal change by the completion of the case, a systematic troubleshooting algorithm is applied to the equipment to ensure that equipment malfunction is not the cause of the decreased EMG signal. If this algorithm is exhausted and no equipment problem is identified, nerve injury is implied. Monitoring can then be used to identify and map out the site of injury on the nerve. The stimulation probe stimulates the nerve at its most distal point, at its laryngeal entry point, and then is progressively run down the nerve in a proximal direction. As soon as the injured segment is identified, the EMG signal falters.
- ♦
With this pattern of distal positive stimulation and proximal negative stimulation, the injured segment of the nerve can be identified, allowing the surgeon to potentially treat the injured segment. For example, if there is a suture adjacent to the nerve that is entrapping the nerve, the suture can be removed. At the very least, it allows the surgeon to understand exactly what segment of the nerve was injured and to review the surgical case to determine what maneuver led to the injury. In this way it provides an excellent learning opportunity for the surgeon.
- ♦
The second main consideration with decreased EMG activity after the nerve segment injury site is mapped out is to incorporate this information when considering operating on the second side that day. Certainly, surgery on a normally functioning nerve is required in many settings in advanced and revision cancer surgery, but it is important for the surgeon and patient to be aware that this is the patient’s only functioning nerve.
- ♦
In many cases of transient neurapraxia, simply delaying the case a matter of several weeks will allow the first side to resume function, which can be confirmed with postoperative laryngoscopy; second side surgery can be offered once the first side has resumed normal function. We feel that neural monitoring should be applied to all cases of thyroid and parathyroid surgery because it is not always possible to identify which cases will involve problematic nerve dissections before the start of the case. Certainly advanced cancer, extensive paratracheal nodal disease, difficult neck morphology, and large goiters, when identified preoperatively, may suggest a difficult case, but sometimes small glands and thin necks can result in very complex and difficult ligament of Berry dissections and result in neurapraxia. Because of this lack of predictability and because of the learning curve, which is substantially steepened through routine application of neural monitoring, we recommend this routine application in all cases of thyroid and parathyroid surgery.
- ♦
Initial endotracheal tube placement can be confirmed with the finding of respiratory variation on the monitor once the intubation-related dose of paralytic agent has worn off but before the inhalational plane is too deep. Once the patient is positioned, respiratory activity can be identified on the bilateral electrodes of the endotracheal tube as small waveforms of 30 to 70 mV; we term this respiratory variation . Its presence in a positioned (i.e., extended) patient implies appropriate endotracheal tube placement with electrodes at the level of the vocal cords.
- ♦
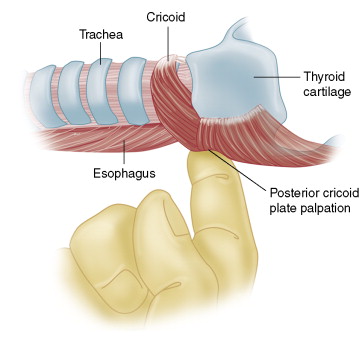
If there is a loss of signal during the case, the very first troubleshooting step is the identification of laryngeal twitch. This is done with an index finger behind the posterior plate of the cricoid cartilage to assess during nerve stimulation the palpable twitch of the posterior cricoarytenoid muscle ( Fig. 6-7 ). Once a laryngeal twitch is established, nerve identification and lack of nerve injury are assured and a troubleshooting algorithm can be applied to the monitoring equipment.