Central Nervous System Infections Unrelated to Neurosurgery
Meningitis and encephalitis are part of a clinical spectrum of CNS disorders causing fever and meningismus. Encephalitis may manifest with signs and symptoms of meningeal inflammation, but is distinguished by the predominance of alterations of consciousness and neurologic deficits. Initial evaluation generally involves head CT to rule out intracranial bleeding in addition to brain MRI and lumbar puncture (assuming no contraindication). Cerebrospinal fluid (CSF) studies should be tailored to specific host factors, epidemiologic exposures, and clinical presentation, but should generally include a cell count with differential, glucose, protein, Gram stain, cryptococcal antigen, fungal culture, and bacterial culture. Noninfectious causes of meningitis include carcinomatous meningitis, nonsteroidal antiinflammatory medications, TMP/SMX, and serum sickness (e.g., associated with antilymphocyte gammaglobulin or intravenous immunoglobulin [IVIG]).
Empiric treatment for suspected bacterial meningitis should include antimicrobial agents that penetrate the blood-brain barrier and enter the CSF, such as ceftriaxone, ampicillin, and vancomycin.
95 This regimen provides coverage against the most common causes of bacterial meningitis, including penicillin-resistant pneumococci and listeriosis. The combination of vancomycin and TMP/SMX may be used in patients allergic to penicillins.
95 Cefepime or meropenem should be used instead of ceftriaxone in patients at risk for
P. aeruginosa meningitis (e.g., neutropenia, neurosurgery within the past 2 months, allogeneic HSCT, prior history of
P. aeruginosa infection). Meropenem should also provide appropriate coverage against
Listeria. Conflicting results have been reported regarding the use of dexamethasone as an adjuvant therapy in the management of bacterial meningitis. Data
from a large meta-analysis found dexamethasone to not provide significant reductions in death or neurologic sequelae, although a statistically significant reduction in hearing loss was observed among surviving patients.
97 ISDA guidelines for the management of bacterial meningitis support the incorporation of adjuvant dexamethasone in pediatric patients with
H. influenzae type B meningitis and in adult patients with pneumococcal meningitis.
95Encephalitis in patients with cancer is most commonly caused by HSV. Intravenous acyclovir should be considered as empiric therapy for HSV in patients with suspected encephalitis (fever, mental status changes, CSF pleocytosis, and focal changes on EEG or MRI, especially in the temporal lobes). CSF studies should include PCR for HSV and CSF cytology. PCR for arboviruses should be considered in patients with exposure to endemic areas. The CSF should also be sent for nucleic acid amplification, adenosine deaminase level, and culture for tuberculosis in patients with known or suspected encephalitis. Patients with severe impairment of cellular immunity (e.g., allogeneic HSCT recipient, advanced AIDS) should be evaluated with PCR for CMV, VZV, HHV-6, and toxoplasmosis as well as culture for Nocardia. Most cases of encephalitis occur in HSCT patients and are due to reactivation of latent viral, bacterial, or parasitic infections: herpesviruses (HSV, VZV, CMV, EBV, human herpes virus [HHV]-6), adenovirus, mycobacteria, and Toxoplasma gondii.
Herpes viruses
HSV meningoencephalitis has been associated with older age, steroid therapy, and brain irradiation. The diagnosis is usually made by viral PCR from the CSF and enhancement of the temporal lobe on MRI. Intravenous acyclovir should be considered as empiric therapy for HSV.
96 Other, less common herpesvirus infections can also be diagnosed by PCR of viral DNA in the CSF. Detection of high levels of EBV DNA should raise suspicion of EBV-related lymphoproliferative disorder, but primary EBV encephalitis and myelitis have been described.
98,99 HHV-6 has been associated with a characteristic syndrome in HSCT patients consisting of confusion, lethargy, fever, rash, and hippocampal enhancement on T2-weighted MRI FLAIR images.
100 The diagnosis is made by detection of HHV-6 DNA in the CSF by PCR. Ganciclovir or foscarnet may be used in treating HHV-6 infections.
101 Adenoviral encephalitis in HSCT patients has been reported as part of disseminated adenoviral infection and treatment with cidofovir may be attempted.
96Other uncommon but important causes of meningoencephalitis are due to new primary infections rather than reactivation. Advanced age and cancer are risk factors for encephalitis from West Nile virus (WNV) transmitted by mosquitoes and for which there is no proven therapy.
102
Brain Abscess
Brain abscesses that develop during neutropenia are typically caused by fungi (commonly
Aspergillus and
Candida).
103 Bacterial abscesses may occur as a local extension of infection of the sinuses or dental caries and are often caused by a mixed aerobic and anaerobic flora (streptococci,
Staphylococcus, Bacteroides). Other causes of CNS abscesses in patients with impaired cellular immunity include toxoplasmosis, nocardiosis, cryptococcosis, and mycobacterial infections.
103Noninfectious etiologies include CNS malignancies, including secondary lymphomas and EBV-associated posttransplant lymphoproliferative disease (PTLD) in patients with impaired cellular immunity. Given the broad differential diagnosis of new focal CNS lesions in the highly immunocompromised patient, a brain biopsy is recommended if feasible. Cultures and stains should include bacteria, fungi, mycobacteria, and Nocardia species. Serum galactomannan, CSF galactomannan, and beta-d-glucan testing are useful to facilitate a diagnosis of CNS aspergillosis.
Brain abscesses usually manifest with headache, focal neurologic findings, or seizures.
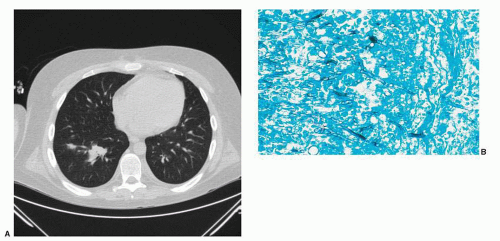
104,105 MRI typically shows single or multiple lesions with edema and ring enhancement. Manifestations of CNS aspergillosis include focal seizures, hemiparesis, cranial nerve palsies, and hemorrhagic infarcts due to vascular invasion.
106 Aspergillus brain abscesses are typically multiple, hypodense, and nonenhancing with little mass effect. CT scans with contrast enhancement may initially fail to reveal focal lesions, but subsequently evolve focal ring-enhancing or hemorrhagic lesions.
107Initial therapy with ceftriaxone plus metronidazole is advised in immunocompetent patients with a bacterial brain abscess.
3,104,105,108 Patients with prolonged neutropenia should be treated with the combination of meropenem or cefepime plus metronidazole and voriconazole
3,104 Adjustments may be needed for patients requiring certain antiseizure agents (e.g., phenytoin) due to the potential for significant drug
–drug interactions with voriconazole (as well as itraconazole and posaconazole).
109 Empiric high-dose TMP/SMX (trimethoprim component: 5 mg/kg every 8 hours) should be considered to cover toxoplasmosis and nocardiosis in allogeneic HSCT recipients and patients with severe T cell impairment. Retrospective analysis of CNS aspergillosis treated with voriconazole as either primary or salvage therapy indicated that 34% had a complete or partial response
110 and compares favorably to previous reports in which frequency of successful responses to amphotericin B in CNS aspergillosis was close to zero.
111
Cryptococcosis
Lymphoid malignancy and corticosteroid therapy are major risk factors for cryptococcal infection.
112 Host defense against cryptococcal infection is dependent on T cell immunity. Isolated neutropenia is rarely associated with cryptococcal infection. The principal portal of entry of this organism is by inhalation. Spread to the blood and then to the central nervous system is a prerequisite for subsequent development of cryptococcal meningitis. Although meningitis is the most common presentation of cryptococcal infection, other manifestations include pneumonia, fungemia, cutaneous infections, and visceral dissemination. Visual loss may be a consequence of endophthalmitis (a space-occupying lesion in the visual pathway), direct invasion of the optic nerve, and elevated intracranial pressure.
113The IDSA recommends a regimen of amphotericin B (0.7 to 1 mg/kg daily) plus 5-fluorocytosine (100 mg/kg daily) for the first 2 weeks, followed by life-long maintenance fluconazole therapy in AIDS-associated cryptococcal meningitis.
114 In the absence of modern randomized studies, the same induction regimen is recommended in non-AIDS-associated cryptococcal meningitis, followed by fluconazole for at least 10 weeks or until immunosuppressive agents have been discontinued.
114 Reduction of the dosage of 5-fluorocytosine may be considered to minimize delay of myeloid recovery in neutropenic patients.
Toxoplasmosis
Reactivation of previously acquired infections is responsible for the majority of opportunistic CNS infections caused by
T. gondii. The organism can be acquired by ingestion of undercooked meat or through contact with feline feces. CNS toxoplasmosis is typically associated with disseminated infection, and risk factors include therapy with corticosteroids, alemtuzumab, cytotoxic agents, and/or radiation therapy, and poorly controlled malignancy. Although toxoplasmosis is an uncommon complication of HSCT, nearly all occurrences are associated with seropositivity prior to transplantation.
115 It tends to occur in the presence of moderate to severe GVHD with a median day of onset of disease at 64 days post-HSCT.
116Common clinical findings for CNS toxoplasmosis include altered mental status, coma, seizures, cranial nerve abnormalities, and motor weakness.
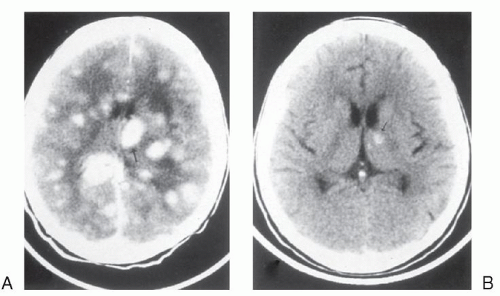
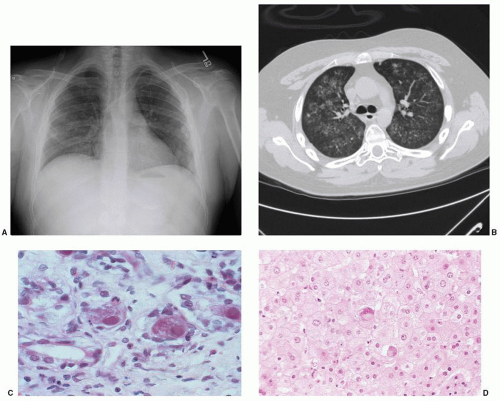
117 MRI typically shows two or more lesions that may be ring-enhancing (
Fig. 69.4). The differential diagnosis includes bacterial infection, invasive aspergillosis, nocardiosis, and malignancy. Differentiating toxoplasmosis from lymphoma is particularly difficult; [18F] fluorodeoxyglucose positron emission tomography typically shows increased metabolism in lymphoma.
118 CSF in toxoplasmosis is usually normal but mononuclear pleocytosis and elevated protein levels may occur. In addition to CNS involvement, toxoplasma may also present with myocarditis, interstitial pneumonitis, culture-negative sepsis, and hemophagocytic syndrome. Definitive diagnosis of toxoplasmosis relies on demonstration of tachyzoites and cysts in histopathologic sections, but PCR testing of serum and CSF may facilitate earlier diagnosis.
117,119 Oral sulfadiazine 1 to 1.5 g every 6 hours plus pyrimethamine (loading dose of 200 mg, followed by 75 mg daily) is the initial treatment of choice for toxoplasmosis. Folinic acid (10 to 20 mg daily) should be administered to reduce myeloid toxicity. At 4 to 6 weeks after resolution of symptoms and signs of infection and radiologic improvement, switching to a maintenance regimen (sulfadiazine 0.5 to 1 g four times daily plus pyrimethamine 50 mg/day) is reasonable. Maintenance therapy should be continued for the duration of immunosuppression and until radiologic resolution. Clindamycin and primaquine may be used instead, after verifying normal glucose-6-phosphate dehydrogenase activity in patients intolerant of sulfonamides. Atovaquone may also be used after exhausting other options.
96
Dementias
Progressive multifocal leukoencephalopathy (PML) is a demyelinating disease associated with lytic infection of oligodendrocytes by the human polyomavirus JC virus.
40 Patients may develop rapidly progressive dementia as well as focal motor neuropathy or cerebellar degeneration. This disease is most commonly seen in patients with advanced AIDS. However, PML is also seen in severely immunocompromised persons with hematologic malignancies and in HSCT recipients. Several reports have linked the use of rituximab with the development of PML and prompted an FDA advisory regarding a possible association.
120 PML has also been reported with other immunosuppressants and biologics including belatacept,
121 brentuximab,
122 efalizumab,
123 fludarabine,
124 infliximab,
125 and mycophenolate.
126 Brain MRI typically reveals unilateral or bilateral white matter disease without mass effect or enhancement. Diagnosis is generally confirmed by detection of the JC virus in spinal fluid by PCR (90% sensitivity) or by brain biopsy. There is no established therapy for PML and the prognosis is poor.
Infections Related to Neurosurgical Procedures and/or Devices
Neurosurgical procedures such as resection of tumor, insertion of a shunt for hydrocephalus, or insertion of an Ommaya reservoir each carry a risk for infectious complications. Infection risk is further increased with use of steroids and brain irradiation. Early postoperative infections after placement of intraventricular devices are usually caused by skin flora: coagulase-negative staphylococci, S. aureus, streptococci, and Propionibacterium acnes. Enterobacteriaceae and P. aeruginosa account for approximately 10% of infections. Coagulase-negative staphylococci and P. acnes usually cause indolent late postoperative infections.
Infection of a shunt or an Ommaya reservoir may manifest with malfunction of the device, fever, or altered mental status. Overt signs of meningitis, such as meningismus and photophobia are often absent. CT imaging may suggest meningitis, ventriculitis, or a brain abscess if the device is infected at the proximal end. Evaluation of the CSF is required for diagnosis confirmation. Infections occurring in the distal region of the device may manifest as a soft tissue infection. In cases of ventriculoatrial shunts, distal infections may cause persistently positive blood cultures, thrombophlebitis, endocarditis, or septic pulmonary emboli. Distal ventriculoperitoneal shunt infections are associated with peritonitis and intraabdominal collections. Removal of the entire device plus systemic antibiotics is the most effective approach to eradicate infection. Use of parenteral and intraventricular instillation of antibiotics without hardware removal has demonstrated variable success, and recurrence of infection is common, particularly those caused by
S. aureus.
127 Antibiotic therapy should be tailored to the specific pathogen isolated. In acutely ill patients with meningitis suspected to be related to prior neurosurgery, empiric therapy with parenteral vancomycin should be administered to cover
Staphylococcus,
Streptococcus, and
Propionibacterium species in combination with an agent with activity against Enterobacteriaceae and
P. aeruginosa such as ceftazidime or meropenem.
95