INTRODUCTION
SUMMARY
The monocyte is a spherical cell with prominent surface ruffles and blebs when examined by scanning electron microscopy. As the monocyte enters the tissue and differentiates into a macrophage, the cell volume and number of cytoplasmic granules increase. Cell shape varies, depending on the tissue in which the macrophage resides (e.g., lung, liver, spleen, brain). A characteristic feature of macrophages is their prominent electron-dense membrane-bound lysosomes, which can be seen fusing with phagosomes to form secondary lysosomes. The latter contain ingested cellular and noncellular material in different stages of degradation. A broad range of surface receptors for many ligands, including the Fc portion of immunoglobulin, complement proteins, cytokines, chemokines, lipoproteins, and others, are on the cell surface. Macrophages differ in appearance, biochemistry, and function based on the environment in which they mature from monocytes. These differences are exemplified by the diversity among dendritic cells of lymph nodes, histiocytes of connective tissue, osteoclasts of bone, Kupffer cells of liver, microglia of the central nervous system, and macrophages of the serosal surfaces, each fashioned to meet the local needs of the mononuclear phagocyte system, which plays a role in inflammation and host defense against microbes. Modern cell biologic methods refined our knowledge of surface receptors, endocytosis, and lysosomal degradation, with emphasis on membrane flow and secretion. These pioneering studies culminated in the discovery of dendritic cells as potent, specialized antigen-presenting cells. Subsequent development of monoclonal antibodies and molecular cloning of surface proteins and cytokines, followed by microarray analysis and genomics, provided the sensitive and specific tools to analyze macrophage functions in vitro and in vivo. These studies have brought insights into macrophage cytotoxic and antimicrobial activities and, to a lesser extent, their trophic, homeostatic functions in the body. Macrophages play a major role in innate as well as adaptive immunity.
Acronyms and Abbreviations
APC, antigen-presenting cell; CD, cluster of differentiation; CR, complement receptor; CSF, colony-stimulating factor; DC, dendritic cell; EGF, epidermal growth factor; EGF-TM7, epidermal growth factor–seven transmembrane; EMR2, epidermal growth factor–like module containing mucin-like hormone receptor–like 2; FcR, Fc receptor; GM-CSF, granulocyte-monocyte colony-stimulating factor; GPCR, G-protein–coupled receptor; HLA, human leukocyte antigen; IBD, inflammatory bowel disease; IFN, interferon; Ig, immunoglobulin; IL, interleukin; IMP, intramembrane particle; IRAK, interleukin receptor-associated kinase; LFA, lymphocyte function–associated antigen; LPS, lipopolysaccharide; m-ϕ, macrophage; MARCO, macrophage receptor with collagenous structure; M-CSF, macrophage colony-stimulating factor; MHC, major histocompatibility complex; MPO, myeloperoxidase; NF, nuclear factor; NLR, NOD-like receptor; NOD, nucleotide-binding oligomerization domain; PI3K, phosphatidylinositol 3-kinase; PS, phosphatidylserine; SR, scavenger receptor; TGF, transforming growth factor; TLR, toll-like receptor.
MONONUCLEAR PHAGOCYTE SYSTEM OVERVIEW
Modern study of mammalian phagocytes began with Metchnikoff in the 19th century. An understanding of the ontogeny, kinetics, and function of phagocytic cells in animals led to the concept of the mononuclear phagocyte system.1,2 Kinetic studies indicate that marrow monoblasts and monocytes develop from the common myeloid progenitor, a derivative of the hematopoietic stem cell, and that tissue macrophages develop from monocytes that have migrated from the blood pool in response to chemotactic stimuli (Table 67-1 and Chap. 18). Tissue macrophages share many functional characteristics, such as phagocytic and microbial killing capabilities and adherence to glass or plastic surfaces in vitro. Vascular endothelium, reticular cells, and dendritic cells of lymphoid germinal centers usually are not included in the mononuclear phagocyte system, although the now obsolete term reticuloendothelial system3 denoted those cells as playing some complementary part with mononuclear phagocytes. In addition to developing the multitude of types of tissue macrophages, monocytes can differentiate into myeloid-derived dendritic cells.4,5
| Marrow | Tissues |
|---|---|
| Monoblasts | Liver (Kupffer cells) |
| Promonocytes | Lung (alveolar macrophages) |
| Monocytes | Connective tissue (histiocytes) |
| Macrophages | Spleen (red pulp macrophages) |
| Blood | Lymph nodes |
| Monocytes | Thymus |
| Body cavities | Bone (osteoclasts) |
| Pleural macrophages | Synovium (type A cells) |
| Peritoneal macrophages | Mucosa-associated lymphoid tissue |
| Inflammatory tissues | Gastrointestinal tract |
| Epithelioid cells | Genitourinary tract |
| Exudate macrophages | Endocrine organs |
| Multinucleate giant cells | Central nervous system (microglia) |
| Skin (histiocyte/dendritic cells) |
STRUCTURE
The blood monocyte is a medium to large motile cell that can marginate along vessel walls and has a propensity for adherence to surfaces. Monocytes respond to inflammation and chemotactic stimuli by active diapedesis across vessel walls into inflammatory foci, where they can mature into macrophages, with greater phagocytic capacity and increased content of hydrolytic enzymes. Free macrophages also are present in mammary glands, alveolar spaces, pleura, peritoneum, and synovia. The somewhat less-motile fixed-tissue macrophages are found in different tissues and serous cavities. The functions of mononuclear phagocytes include phagocytosis, killing, and digestion of microorganisms, particulate material, or tissue debris; secretion of chemical mediators and regulators of the inflammatory response; interaction (as dendritic cells) with antigen and lymphocytes in the generation of the immune response; cytotoxicity, such as killing of some tumor cells; and other functions specific for macrophages of particular tissues.
The development of techniques to isolate monocytes from blood of adult human subjects led to the discovery that monocytes are heterogeneous with regard to cell volumes. Isolation of purified monocytes by adherence to glass substrates or to gelatin-coated flasks or by centrifugal elutriation reveals distinct populations of monocytes.1,2 In addition to the usual 12- to 15-μm diameter (when measured on a dried blood film) monocyte, so-called regular monocytes, a somewhat smaller cell that is less active than its larger, more mature counterpart has been identified. This cell is referred to as a small immature monocyte, but its functional significance is not clear.
Monocytes continuously emigrate from the blood into tissue, with a half-life in the blood of approximately 1 day in mice.6 Nondividing monocytes can be induced to differentiate into dendritic like cells in vitro. However, this process requires culture of the cells for 7 to 10 days with exogenous cytokines, typically interleukin (IL)-4 and granulocyte-monocyte colony-stimulating factor (GM-CSF).7 The major lineage regulator of nearly all macrophages is monocyte/macrophage colony-stimulating factor (M-CSF; also termed CSF-1) and its receptor (M-CSF R). The M-CSF R is a class III transmembrane tyrosine kinase receptor, which is expressed on most mononuclear phagocytes.8 In the presence of endothelial cells grown on an extracellular matrix, monocytes differentiate along two distinct pathways: toward dendritic cells or macrophages. Monocytes that migrate across endothelium in an abluminal to luminal direction differentiate into dendritic cells. In contrast, monocytes that remain in the subendothelial matrix differentiate into macrophages.
Monoblasts and promonocytes are the precursors of monocytes, bearing finely dispersed nuclear chromatin and nucleoli when observed in the stained film of the marrow. The monoblast is a very-low-prevalence marrow cell, indistinguishable by light microscopy from the myeloblast. Promonocytes are 12 to 18 μm in diameter (as measured on dried blood films) and have characteristic deeply indented, irregularly shaped nuclei with condensed chromatin, and numerous cytoplasmic microfilaments.
In animal studies, a small percentage of marrow cells are phagocytic, synthesize DNA, adhere to glass surfaces, and contain nonspecific esterases.9 These cells have been referred to as promonocytes and are considered as intermediate between monoblasts and the monocytes of the blood.9 Cytochemical studies identify the promonocyte in normal human marrow. Promonocytes have deeply indented and irregularly shaped nuclei and bundled and scattered single filaments in the cytoplasm. These morphologic features distinguish the promonocyte from the progranulocyte.10,11 Peroxidase is present throughout the cell secretory apparatus in all cisternae of the rough-surfaced endoplasmic reticulum, the Golgi complex, associated vesicles, and all immature and mature granules. Cytochemical reaction products for acid phosphatase and arylsulfatase also are deposited throughout the secretory apparatus of the promonocyte.
The morphology of monocytes has been investigated by light and phase-contrast optics,12 scanning and transmission electron microscopy, and freeze-fracture and freeze-etch procedures.13
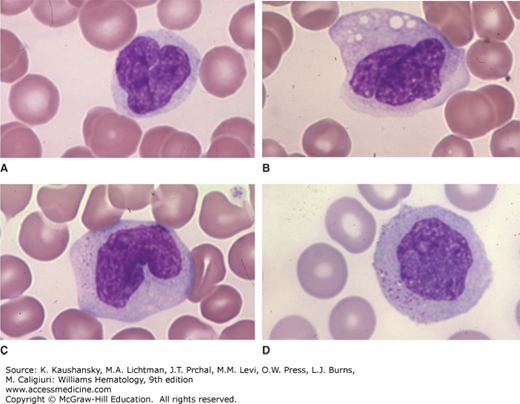
On the stained blood film the monocyte has a diameter of 12 to 15 μm (Fig. 67–1). The monocyte nucleus occupies approximately half the area of the cell and usually is eccentrically placed. The nucleus most often is reniform, but may be round or irregular. It contains a characteristic chromatin net with fine strands bridging small chromatin clumps. Chromatin aggregates are arranged along the internal side of the nuclear membrane. The cytoplasm is spread out, stains grayish-blue with Wright stain, and contains a variable number of fine, pink-purple granules, which at times are sufficiently numerous to give the entire cytoplasm a pink hue. Clear cytoplasmic vacuoles and a variable number of larger azurophilic granulations often are encountered in these cells.
Figure 67–1.
Blood films. This composite shows four examples of normal monocytes with different nuclear configurations. A. In this case, the nucleus is contorted on itself and the nuclear-to-cytoplasmic ratio is a bit higher than the average case. B. Another contorted nucleus with a lower nuclear-to-cytoplasmic ratio. Scattered vacuoles are common in monocytes collected in ethylenediaminetetraacetic acid (EDTA)-anticoagulated blood before film preparation. C. Characteristic reniform nuclear shape. D. Circular nuclear shape. Azurophilic granules are evident in the cytoplasm of monocytes. (Reproduced with permission from Lichtman’s Atlas of Hematology, www.accessmedicine.com.)
The monocyte nucleus has a distinct chromatin pattern on a cloudy background when examined by phase-contrast microscopy. The cytoplasm is clear gray. Mitochondria are extremely fine and occasionally form a small, juxtanuclear rosette surrounding the centrosome. The phase-dense cytoplasmic granules, varying in number, are generally at the limit of resolution of light microscopy and appear as fine intracytoplasmic dust. Monocytes contain several types of cytoplasmic vacuoles. The reniform nucleus with a juxtanuclear depression filled by a centrosome and its active undulating movement similar to that of other leukocytes are characteristic of the monocyte. The locomotion of the monocyte has the same pattern of undulating cytoplasmic veils seen in macrophages. The monocyte generally assumes a triangular shape as it moves, with one point trailing behind and the other two points advancing before the cell. Blood monocytes undergo adherence and cytoplasmic spreading following attachment to glass surfaces.14 The extent of spreading increases in the presence of antigen–antibody complexes, certain divalent metals, and proteolytic enzymes.14,15 The spread form of the monocyte reveals that the nucleus and granules are located centrally and the abundant hyaloplasm is in the periphery of the cell, terminating in a fringed border that displays undulating movement. The small monocyte may be difficult to distinguish from the large lymphocyte when examined by phase-contrast microscopy.
A striking feature on phase-contrast microscopy is the ruffled plasma membrane that forms prominent phase-dense folds at the cell surface and edges. Some cells have a dense thickening at the edge of the cytoplasm, with microextensions on the thickened edge.
The monocyte surface has very prominent ruffles and small surface blebs.16,17 Extensive ruffling on the monocyte plasma membrane is of functional significance. The monocyte is both motile and phagocytic, and these functions require physical contact with particles or cell surfaces. Reduction in the radius of curvature of the cell surface by formation of ruffles or microvilli may reduce repulsive forces when surface negative-charge groups on the cell approach and contact a negatively charged substratum or cell. In addition, redundancy of the cell membrane may provide reserve membrane required for locomotion and phagocytosis.
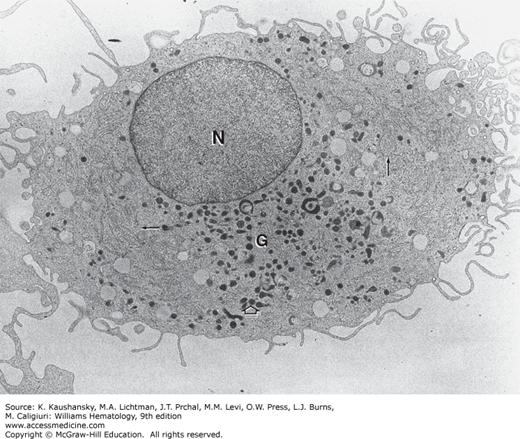
The nucleus of the monocyte contains one or two small nucleoli surrounded by nucleolar-associated chromatin (Fig. 67–2).18 The cytoplasm contains a relatively small quantity of endoplasmic reticulum and a variable quantity of ribosomes and polysomes. The mitochondria are numerous, small, and elongated. The Golgi complex is well developed and is situated about the centrosome within the nuclear indentation. Centrioles and filamentous centriolar satellites are often visualized in this region. Microtubules are numerous, and microfibrils are found in bundles surrounding the nucleus. In cultured macrophages, collections of microfilaments are present underneath the plasma membrane near sites of cell attachment either to a substratum or to phagocytosable particles.19 The cell surface is characterized by numerous microvilli and vesicles of micropinocytosis. The cytoplasmic granules resemble the small granules found in the granulocytic series, measuring approximately 0.05 to 0.2 μm in diameter. They are dense and homogeneous and are surrounded by a limiting membrane. These granules, as with the lysosomal granules of other leukocytes, are packaged by the Golgi apparatus after their enzymatic content has been produced by the ribosomal complex of the cell.10,11 These cytoplasmic granules contain acid phosphatase and arylsulfatase and, therefore, are primary lysosomes. After endocytosis, lysosomes fuse with the phagosome, forming secondary lysosomes. Some monocyte granules stain positive for peroxidase, whereas others are peroxidase negative.10,11
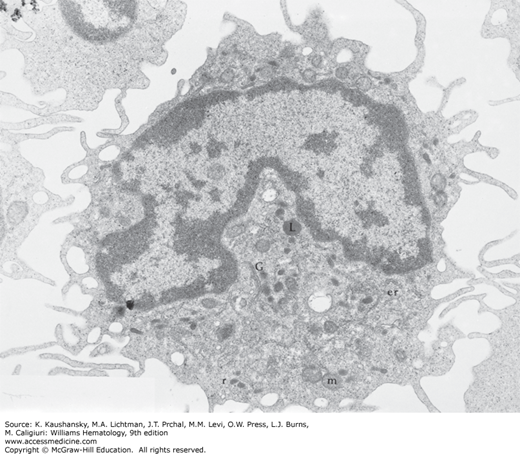
Figure 67–2.
Transmission electron micrograph of a monocyte. The eccentric reniform nucleus has a thinly dispersed chromatin pattern. The Golgi complex (G) is in a juxtanuclear position. Small electron-dense granules can be seen evolving in the Golgi complex. Small amounts of rough endoplasmic reticulum (er) and polyribosomes (r) are present, particularly about the cell periphery. Mitochondria (m) are concentrated in the region of the Golgi apparatus; they also are scattered in the cell periphery. Lysosomes (L) are small, electron-dense granules surrounded by a limiting membrane. The irregular ruffled cell margin is apparent with numerous microprojections (×24,000).
In this technique, a cell suspension is frozen, placed in a high-vacuum chamber, and struck with a blunt edge, thus producing a fracture that propagates through the frozen specimen. The utility of the procedure comes from the remarkable finding that when the fracture encounters a cell, the fracture tends to propagate along the interior of the plasma membrane and thus split the lipid bilayer into its two constituent layers. After fracture, the specimen is coated with platinum, which is electron dense when viewed with transmission electron microscopy. All cell types examined thus far by the freeze-fracture technique reveal intramembrane particles (IMPs) as the predominant topographic feature of the interior of the bilayer. Studies of the erythrocyte show that at least some particles contain intercalated membrane proteins, and this is assumed to be the case for nucleated cells as well. The distribution of IMPs is dramatically altered in a number of cell systems by physiologic stimuli, for example, hormonal stimulation.
Profound changes in the distribution of IMPs on mononuclear phagocytes occur following binding of antibody-coated erythrocytes.13 Because redistribution of IMPs also occurs in some nonphagocyte Fc receptor (FcR)–bearing cells13 and after exposure to aggregated immunoglobulin (Ig) G, this alteration in IMPs presumably reflects interaction with FcR. Freeze-etch electron micrographs of the monocyte show nuclear pores traversing both lamellae of the nuclear membrane and contours of cytoplasmic lysosomes and mitochondria (Fig. 67–3).
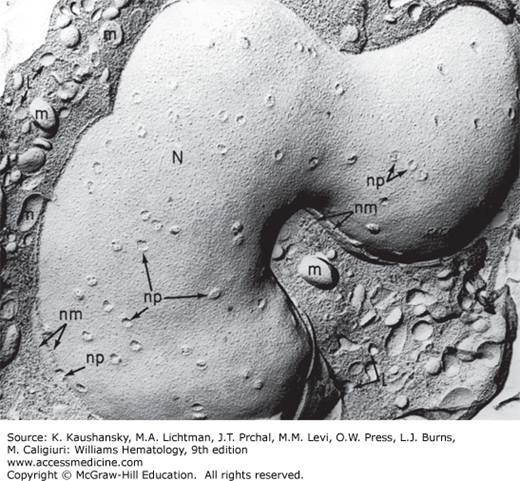
Figure 67–3.
Freeze-etch electron micrograph of a monocyte. Fracture plane displays the large nucleus (N), with multiple nuclear pores (np) and the two lamellae of the fractured nuclear membrane (nm) evident in some regions. Membrane and cleaved surfaces of mitochondria (m) and lysosomal granules (L) can be identified in the cytoplasm.
Table 67–2 compares the hydrolytic enzyme contents of monocytes, neutrophils, and lymphocytes. Monocytes also give a weak but positive periodic acid–Schiff reaction (for polysaccharides) and Sudan black B reaction (for lipids). Nonspecific esterase20,21,22 is frequently used as a marker for monocytes. Monocyte esterases are inhibited by sodium fluoride, whereas the esterases of the granulocytic series are not. The nonspecific esterase reaction is positive in promyelocytes and myelocytes; therefore, analysis of fluoride inhibition is necessary to distinguish marrow monocytes from early myelocytes. Monocyte granules, although heterogeneous in size (0.3 to 0.6 μm), are not separable into populations by routine electron microscopic criteria. Identification of monocyte granule populations has depended on subcellular localization of monocyte enzymes by electron microscopic cytochemistry.10 Human marrow promonocytes and blood monocytes contain granules that comprise two functionally distinct populations.10,11 One population contains the enzymes acid phosphatase, arylsulfatase, and peroxidase. These granules are modified primary lysosomes and are analogous to the azurophil granules of the neutrophil. The monocyte azurophil granule population is heterogeneous in cytochemical reactivity for peroxidase, acid phosphatase, and arylsulfatase.23,24 Moreover, primary granules that are morphologically identical with other vesicles can be identified as lysosomes cytochemically. The other population of monocyte granules lacks alkaline phosphatase23 and is not strictly analogous to the specific granules of neutrophils.
| Chemical | Monocytes | Neutrophils | Lymphocytes |
|---|---|---|---|
| Acid phosphatase | + + | + | + |
| β-Glucuronidase | + + | + | 0 to + |
| Sulfatase | + | + | 0 |
| N-Acetylglucosaminidase | + + | + + | 0 |
| Lysozyme* | ++ | + + | 0 |
| Naphthylamidase | + + | + | 0 to + |
| α-Naphthylbutyrate esterase† | + + | 0 to + | 0 |
| Naphthol AS-D chloroacetate esterase | 0 to + | + + | 0 |
| Peroxidase | + | + + | 0 |
| Alkaline phosphatase | 0 | 0 to + | 0 |
Macrophage characteristics are heralded by a significant increase in cell size, increase in the number of cytoplasmic granules, increase in the heterogeneity of cell size and shape, and increase in the number of cytoplasmic clear vacuoles in comparison to monocytes.
In vitro culture of monocytes purified from adult human blood has provided an opportunity to observe the maturation of these cells into mature macrophages. The macrophages of the pulmonary alveoli, peritoneal and pleural cavities, and inflammatory exudates are hypermature cells that have undergone in vivo stimulation and maturation. This process results in enhanced bactericidal activity1,2 because of augmentation of lysosome number and acid hydrolase content. Macrophages display attributes of morphologic specialization specific to their location and function. The fixed macrophages of the spleen (littoral cells) are involved in the sequestration and destruction of effete or abnormal red cells and exhibit stages of erythrophagocytosis and intracytoplasmic aggregates of ferritin (Chap. 6). The macrophages of the marrow, the “nurse cells” of the erythroblastic island, play a similar role in erythrophagocytosis and iron storage and transfer (Chaps. 5 and 31). Hepatic macrophages (Kupffer cells), found in liver sinusoids, also phagocytize red cells and other cellular elements and are important sites of iron storage. Macrophages of the pulmonary alveoli, the lamina propria of the gastrointestinal tract, and the peritoneal and pleural fluids reflect in their morphology a specific function of phagocytosis of microorganisms, cells, and cellular and noncellular debris, characteristic of the specific organ location.
Most macrophages are 25 to 50 μm in diameter on Wright or hematoxylin-and-eosin–stained films (Fig. 67–4). They have an eccentrically placed reniform or fusiform nucleus with one or two distinct nucleoli and finely dispersed, loosely stranded nuclear chromatin that tend to clump in the nuclear interior and along the internal aspect of the nuclear membrane (Fig. 67–5A). A juxtanuclear clear zone (Golgi complex) is well defined when the Wright stain is used. The cytoplasm shows fine granules and multiple pink-purple, large azurophil granules. The cytoplasmic borders are irregularly serrated. Cytoplasmic vacuoles are present near the cell periphery, reflecting the active pinocytosis in these cells.
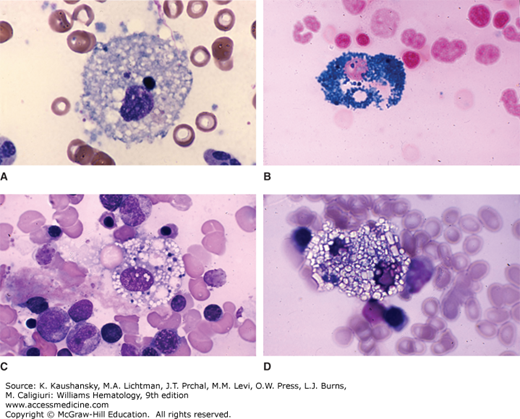
Figure 67–4.
Marrow films. Macrophages. These cells characteristically have a circular, sometimes centrally placed and sometimes eccentrically placed nucleus dwarfed by a very large expanse of cytoplasm. A. Activated macrophage, full of cytoplasmic vacuoles and some residual ingested cellular debris. B. Macrophage stained with Prussian blue showing cytoplasmic iron granules. C. Macrophage with erythrophagocytosis. Note pale red cells (partially dehemoglobinized) undergoing hemolysis and destruction. The highly vacuolated cytoplasm is presumably the site of red cell degradation. D. Macrophage in a patient with cystinosis engorged with cystine crystals. (Reproduced with permission from Lichtman’s Atlas of Hematology, www.accessmedicine.com.)
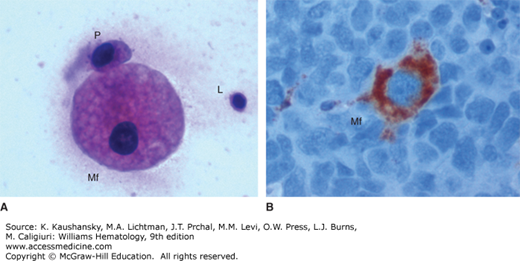
Figure 67–5.
Micrographs of macrophages (Mf). A. Hematoxylin-and-eosin stain of cytology smear (×400) showing a macrophage, a plasma cell (P), and a lymphocyte (L). B. Immunohistochemistry stain for the macrophage marker CD68 of a lymph node (×400). Numerous lymphocytes with blue nuclei surround a macrophage with brown-red cytoplasm. (Used with permission of Dr. Madalina Tuluc, Thomas Jefferson University Hospital, Philadelphia, PA.)
The surface antigen CD68, also known as macrosialin, is commonly used as a macrophage marker. Figure 67–5B shows an immunohistochemistry micrograph of a macrophage in a lymph node. The cytoplasm of the macrophage is intensely positive for CD68, while the surrounding lymphocytes are negative.
On phase-contrast microscopy, living macrophages are large cells with a propensity to adhere to and spread on glass surfaces. Thus, the cell organelles are concentrated within the central portion of the cell and clear veils of hyaloplasm spread about the cell, with intense ruffling of the membrane borders. Vesicles and contractile vacuoles are seen about the cell periphery and in the cell interior. The juxtanuclear clear zone bearing the centrosome and the Golgi complex is particularly dynamic and displays an undulating motion.
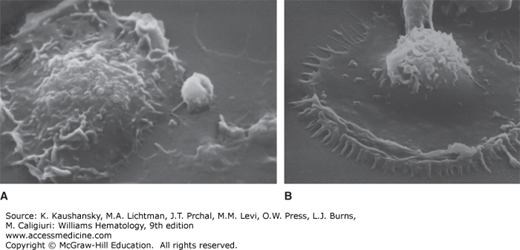
Scanning electron micrographs of macrophages adherent to glass surface show membrane ruffling and pseudopodia (Fig. 67–6). Transmission electron microscopy of monocyte-derived macrophages show a variable degree of differentiation, nuclear “maturity,” ribosomes, mitochondria, and lysosome content, and the nucleus varies in shape from horseshoe to fusiform (Fig. 67–7). Clear spaces between membrane-fixed chromatin aggregates mark the sites of nuclear pores that are relatively abundant on freeze-etch electron micrographs of macrophages and monocytes (see Fig. 67–3). Polyribosomes and scant smooth and rough endoplasmic reticulum are seen about the cell periphery. A well-developed Golgi complex is in a juxtanuclear location. It often is multicentric and contains a concentration of vesicles, some with dense inclusions that mark them as early lysosomes. A relatively constant feature of cells engaged in endocytosis is the large number of microvilli at the cell surface. The degree of development of this surface adaptation is related to the phagocytic activity of the cell and its rate of pinocytosis. The number and size of mitochondria vary with the phagocytic and hence metabolic activity of the cell. Mitochondria tend to be grouped about the region of the Golgi complex, although several usually are seen dispersed about the cell periphery, presumably supplying energy for the active endocytic processes occurring there.
Figure 67–6.
Scanning electron micrograph of cultured macrophages on coverglasses coated with (A) bovine serum albumin (BSA) or (B) with immune complexes (BSA–anti-BSA). The macrophage develops prominent peripheral membrane ruffling and numerous microadhesion points to the surface coated with immune complexes.
The most constant and characteristic ultrastructural features of macrophages are the electron-dense membrane-bound lysosomes that often can be seen fusing with phagosomes to form secondary lysosomes. Within the secondary lysosomes, ingested cellular, bacterial, and noncellular material can be seen in various stages of degradation, often recognizable as degenerating mitochondria or nuclear material. These secondary lysosomes also contain partially degraded material from the late stages of the endocytic process, often appearing as multilamellar lipid bodies. Microtubules and microfilaments are prominent in macrophages. Actin- and myosin-like proteins have been isolated from monocytes and partially characterized. Resting macrophages have irregular cell borders and pseudopodia pushed out in all directions. Their cytoplasm has rough endoplasmic reticulum and Golgi complex in the perinuclear area. Lipid globules, primary lysosomes, and mitochondria are characteristically prominent. Activated monocytes/macrophages are motile cells that extend a leading pseudopod as they move forward.25
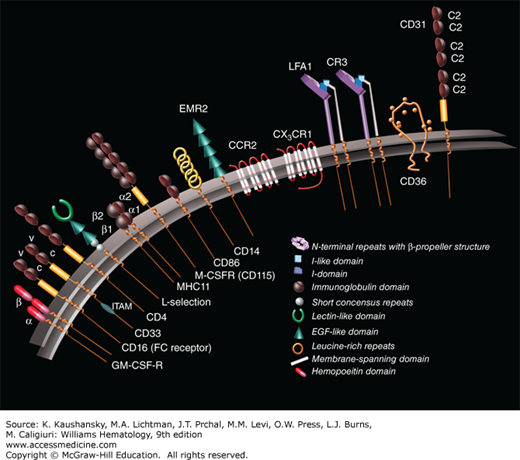
RECEPTORS
Monocyte/macrophage cells have surface receptors that have been characterized by their binding to specific monoclonal antibodies. These receptors (Fig. 67–8) are markers for origin, growth, differentiation,26 activation, recognition, migration, and function of the monocyte/macrophage. Monocytes have been classified into distinct subtypes based on surface expression of CD14 and CD16, molecules that form part of the lipopolysaccharide (LPS) toll-like receptor (TLR) and one of the immunoglobulin FcRs, respectively. These include CD14+-bright/CD16– monocytes, CD14+-dim/CD16+ monocytes, and CD14-dim/CD16+ monocytes. Monocyte heterogeneity was initially divided into the CD14+-bright/CD16-negative cells, which comprise 90 to 95 percent of total circulating monocytes (classical monocyte)27—CD14-bright or dim refer to the fluorescence magnitude of staining using a specific CD14 monoclonal antibody. The minor subset is CD14-dim, CD16-positive, and less phagocytic than the classical monocyte. The classical monocyte produces reactive oxygen species (ROS) and cytokines in response to TLR engagement. The minor subset selectively secretes tumor necrosis factor (TNF)-α, IL-13, and CCL2 in response to viruses and immune complexes containing nucleic acids via TLR-7, TLR-8, MyD88-MEK (myeloid differentiation factor 88–MAPK kinase), and AHD.28 This minor subset, CD14-dim,29 is competent in (SR [scavenger receptor]) function of vascular, intraluminal debris and uptake of immune complexes.30 In addition, their phenotype is related to the ability to produce and secrete select cytokines.31
Macrophages are proficient at endocytosis (both fluid phase and receptor-mediated) and are highly professional phagocytes of particulates of all origin, organic (cellular, microbial) as well as inorganic foreign materials.32 In contrast, when dendritic cells (DCs) mature into antigen-presenting cells (APCs), they have reduced uptake capacity and induce an adaptive immune response or tolerance. Immature DCs display active macropinocytosis and capture exogenous materials for cross-presentation.33 It is convenient to classify plasma membrane uptake receptors as opsonic and nonopsonic TLRs and non–TLR-dependent. The latter category includes a range of SRs34,35 and a family of lectin-like, carbohydrate recognition molecules.36,37 Given the complex ligands presented on the surface of microorganisms and damaged host cells, or generated within the vacuolar system after uptake, these receptors frequently cooperate with one another.
FcRs for IgG are expressed on the surface of mononuclear cells, macrophages, granulocytes, and platelets.38,39 FcRs are divided into three distinct classes: FcRI, FcRII, and FcRIII (Fig. 67–9). These receptors have broad ranges of expression on different cells. The first IgG receptor, FcRI (CD64), is a receptor found on monocytes, macrophages, and activated neutrophils. This receptor binds monomeric IgG through the Fc portion of the molecule. This Ig receptor has increased expression on activated monocytes and macrophages. CD64 allows for receptor-mediated endocytosis of IgG–antigen complexes for presentation to T cells, can trigger the release of cytokines and ROS, and can play a role in granulocyte-mediated antibody-dependent cytotoxicity. The second IgG receptor, FcRII (CD32), is a widely distributed receptor present on many cell types, including monocytes, platelets, neutrophils, B cells, some T cells, and some capillary endothelium. This receptor can bind complexed IgG rather than monomeric IgG. This FcR regulates B-cell function when coengaged with the B-cell receptor for antigen, namely, surface Ig. It also can induce mediator release from myeloid cells and phagocytosis of Ig-coated particles in vitro. Finally, this FcR also can target antigen into presenting pathways. The third IgG receptor, FcRIII (CD16), is expressed by neutrophils, natural killer cells, and tissue macrophages.40 This receptor can bind Ig in immune complexes and Ig bound to cell-surface membranes. It is the main FcR responsible for antibody-dependent cellular cytotoxicity. All three FcRs specifically bind the human IgG subclasses IgG1 and IgG3 (Chap. 75). The interaction of FcR on macrophages with immune complexes results in cell “activation,” with an increase in phagocytosis, superoxide production, and prostaglandin and leukotriene release.
Figure 67–9.
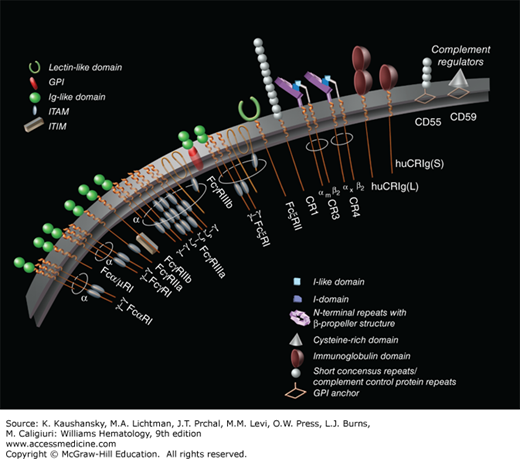
Human Fc receptors and complement. Myeloid cells express a range of classical Fc receptors that initiate a variety of cellular responses, including phagocytosis, antibody-dependent cell-mediated toxicity, antigen presentation, respiratory burst, and release of inflammatory mediators. Immunoglobulin (Ig) subclasses are bound by extracellular domains; signaling via cytoplasmic immunoreceptor tyrosine-based activation motif (ITAM) or immunoreceptor tyrosine-based inhibition motif (ITIM) is mediated by associated membrane-spanning polypeptides. Activation and inhibitory receptors are usually coexpressed on the cell surface and function in concert, determining the magnitude of effector cell responses. Complement receptors (CRs) and membrane regulators are expressed by m-ф-CR. CR1 is broadly expressed by nucleated cells, acting as a “sink” for activated complement. CR3 (CD11b/CD18), a phagocytic receptor for C3bi-coated particles, and CR4 (CD11c/CD18) are β2 integrins, which, together with lymphocyte function-associated antigen (LFA)-1 (CD11a/CD18), mediate adhesion of myeloid cells to endothelium and extracellular matrix and migration. Human CR immunoglobin (huCRIg) are long (L) and short (S) forms of the complement-binding receptor on Kupffer cells that mediate uptake of opsonized bacteria. CD55 and CD59 are glycosylphosphatidylinositol (GPI)-anchored regulators of complement activation. (Used with permission of S. Seif, GraphisMedica, 2014.)
Activation of the complement system results in liberation of numerous ligands that bind to specific receptors on mononuclear phagocytes. Four receptors that bind fragments of the complement component C3 have been identified (see Fig. 67–9).41 Complement receptor (CR) 1 (or CD35) binds dimeric C3bi and is found on both monocytes and macrophages. CR3 (or CD11b) binds the complement fragment C3b. CR3 is a heterodimeric glycoprotein that is composed of two noncovalently linked polypeptides. The α chain of the polypeptide has an Mr of 185,000, and the β subunit has an Mr of 95,000. This receptor and the leukocyte antigens lymphocyte function–associated antigen (CD11a) and alpha-X integrin chain (CD11c) compose a family of heterodimers that share a common β subunit (CD18).42 This family is designated the leukocyte integrin (β2) subfamily.43 These heterodimers are involved in cell–cell interactions, including leukocyte trafficking into the tissues, binding of opsonized particles and plasma proteins, and attachment to various substrates. They also may modulate intercellular adhesion. Elimination of the integrin β2 subunit causes leukocyte adhesion deficiency.44
The classical opsonins, which promote the uptake of particles, are antibody, IgG complexed with antigens, and complement, activated by the classical pathway (antibody-dependent IgM or IgG) or recognized directly via the lectin-carbohydrate–stimulated alternative pathway. Fc and CRs are heterogeneous in structure, expression, and function, activating or inhibiting macrophage responses,45,46 as illustrated in Fig. 67–9. Other opsonins include fibronectin and milk-fat globulin.47 Through their expression of various opsonic receptors, monocytes, macrophages, and DCs perform versatile roles in innate and adaptive immunity,48 in antigen clearance and destruction, in autoimmunity, and in pathogenesis of a range of inflammatory and infectious disorders. Genetic polymorphisms influence the expression and functions of FcRs in homeostasis and disease. Although prominent in host protection, invading microorganisms may be able to exploit, even subvert these receptors to facilitate their entry and survival.49 Opsonic receptors play an important role in clearance of hematopoietic cells, for example, antibody-coated platelets, giving rise to thrombocytopenia, and in therapeutic antibody treatment, for example, to facilitate engraftment. Antibody engineering has provided novel therapeutic agents to minimize undesirable consequences, such as cell activation. The initiation or avoidance of complement activation in particular controls an important effector pathway in tissue injury and repair.
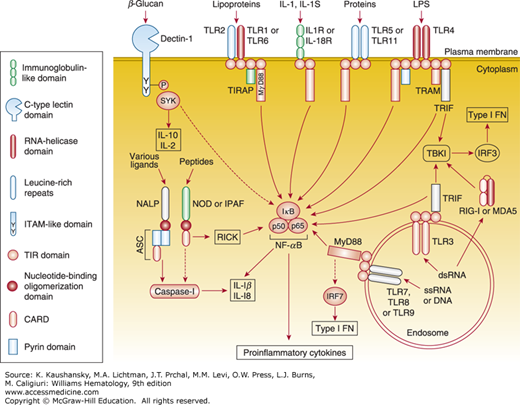
The family of TLRs, identified on macrophages in mammals, is a pattern-recognition receptor that bind structurally conserved molecules derived from microorganisms, including endotoxins (LPS) and viral nucleic acids. TLRs are now considered key molecules responsible for alerting the immune system to the presence of microbial infections. For example, TLR4 is part of a recognition couple for LPS. Pathogen recognition by TLRs activates the innate immune system through the signaling pathway and provokes inflammatory responses, such as cytokine production.50 These are shown schematically in Fig. 67–10 to illustrate their diverse structures and signaling pathways.
Figure 67–10.
The main toll-like receptor (TLR) signaling pathways and adaptor molecules. The pathways that are activated by the different receptors are multiple and complex. For example, TLR signaling involves not only nuclear factor-κB (NF-κB) activation, but also mitogen-activated protein kinases, phosphatidylinositol 3-kinase, and several other pathways that markedly affect the overall biologic response to the activation of TLRs. Dectin-1 (a β-glucan receptor) is shown as an example of various signaling-competent cell-surface pattern-recognition receptors. ASC, apoptosis-associated speck-like protein containing a caspase activation and recruitment domain; CARD, caspase activation and recruitment domain; ds, double-stranded; type I IFN, type I interferon; IFN, interferon; IκB, inhibitor of NF-κB; IL, interleukin; IPAF, interleukin-1β–converting enzyme-protease activating factor; IRF, IFN-regulatory factor; LPS, lipopolysaccharide; MDA5, melanoma differentiation-associated gene 5; MyD88, myeloid differentiation primary response gene 88; NACHT, domain present in NAIP, CIITA, HET-E, and TP-1; NALP, NACHT leucine-rich repeat and pyrin-domain-containing protein; NOD, nucleotide-binding oligomerization domain; RICK, receptor-interacting serine/threonine kinase; RIG-I, retinoic acid-inducible gene I; ss, single-stranded; TBK1, TANK-binding kinase 1; TIRAP, toll/IL-1R (TIR) domain-containing adaptor protein; TRAM, TRIF-related adaptor molecule; TRIF, TIR domain-containing adaptor protein inducing IFN-β; SYK, spleen tyrosine kinase. See text for further details. (Reproduced with permission from Trinchieri G, Sher A: Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol 2007 Mar;7(3):179-190.)
The discovery of TLR has transformed the study of innate immunity, inflammation, and adjuvant actions on APC.51,52,53 Receptor structures, heterogeneity of expression, microbial and endogenous ligands, and signaling have been defined, and knowledge of their regulation has begun to offer agents to manipulate TLR signaling in humans. The discovery of inborn errors, such as the interleukin receptor–associated kinase (IRAK)-4 deficiency,54 and the role of toll-interleukin receptor adaptor protein (TIRAP) function in Plasmodium falciparum infection,55 for example, have illustrated their role in human disease. Several concepts have emerged. From the original studies on LPS recognition and signaling by the multiprotein complex formed by CD14, LPS binding protein, and MD2, and the clarification of the distinct adaptor pathways (MyD88 [myeloid differentiation factor 88], TIRAP/MAL [MyD88 adaptor-like], TRIF [TIR domain-containing adaptor inducing interferon (IFN)-β], and TRAM [TRIF-related adaptor molecule]), the recognition and sensing of TLR ligands have become clear. The tertiary structure of TLR4 has been reported.56 TLRs are expressed either on the plasma membrane of myeloid and other cells, or within the vacuole, especially in the case of TLRs 3, 7, and 9, which are implicated in viral nucleic acid recognition. Crosstalk among nuclear factor (NF) κB, IFN, and mitogen-activated protein kinase (MAPK) kinase pathways has also become apparent.57 TLRs collaborate with other recognition receptors,58 such as dectin-1. Furthermore, a role has been proposed for TLR signaling in nontranscriptional activities, such as the kinetics of phagosome maturation in macrophages.59
The study of lectins and SRs has lagged behind that of the above receptors, but is gaining ground, documenting receptor expression and ligands, mainly in mouse models of inflammation and infection.35,60,61 These receptors are present on macrophages and DCs, and variably on monocytes and neutrophils. They are implicated in the recognition and uptake of microbial and host ligands, and vary in their ability to activate host defense functions. Figure 67–11 and Table 67–3 illustrate the functional attributes of these receptor systems. The mannose receptor is mainly involved in endocytosis, with a predominant intracellular localization.62,63 The multilectin mannose receptor displays dual functions, contributing to the clearance of mannose-terminal lysosomal hydrolases and of neutrophil granule glycoproteins such as MPO, as well as of hormones (e.g., thyroglobulin) and exocrine secretion products (e.g., amylase). It plays a role in the capture and transport of mannose-terminal glycoproteins to targets in spleen (marginal metallophilic macrophages) and in lymph nodes (subcapsular sinus macrophages) that express sulfated receptors for its cysteine-rich domain. The outcome of such targeting is either silent disposal or, if combined with TLR stimulation, induction of an immune response.64 In common with several other nonopsonic receptors, it can play dual, even opposing actions in host protection or in pathogenesis, as shown by ongoing studies in mice.
| Class | Receptor | Microbial Ligands | Endogenous Ligands | Function |
|---|---|---|---|---|
| Scavenger receptors | SR-A I/II | Gram+/– bacteria | Apoptotic cells | Phagocytosis |
| Lipoteichoic acid | Modified low- and high-density lipoproteins (LDL, HDL, apolipoprotein A1, apolipoprotein E) | Endocytosis | ||
| Lipid A | AGE-modified proteins | Foam cell formation | ||
| Neisserial surface proteins | β-Amyloid | Adhesion | ||
| MARCO | Gram+/– bacteria | Marginal zone B lymphocytes | Adhesion |