9 Stool and Blood Sampling for Early Detection of Colorectal Cancer
Introduction
Colorectal cancer is the third most common malignancy in the United States and the third leading cause of cancer deaths amongst all men and women.1 Overall, colorectal cancer has an incidence of 61.3 in 100,000 men and 44.9 in 100,000 women. The lifetime risk for an individual to develop colorectal cancer in the United States is approximately 6%. In an attempt to decrease the incidence of cancer diagnoses and its complications and cancer-related deaths, new methods of genetic screening are being investigated for the detection of premalignant colonic lesions as well as for earlier diagnosis of colorectal tumors.
The current recommendation for the screening of sporadic colorectal cancer consists of noninvasive and invasive measures that should begin at age 50. Screening options are outlined in Box 9-1.2 In general, annual fecal occult blood test (FOBT) in combination with sigmoidoscopy every 5 years is the most common method for screening. In addition, to assess the entire length of colon and rectum, colonoscopic evaluation should be performed every 10 years. Despite evidence that annual FOBT screening has resulted in a reduction of cancer-related deaths from colorectal cancer over a decade, no studies have proved a concurrent reduction in the incidence of cancer. This is primarily due to the lack of sensitivity of these tests for detecting many dysplastic adenomas and early-stage tumors.3–5
Box 9-1 Current Adenomatous Polyp and Colorectal Cancer Screening Options Beginning at Age 50
Data from Smith RA, Cokkinides V, Eyre HJ: American Cancer Society guidelines for the early detection of cancer, 2006. CA Cancer J Clin. 56(1):11–25; quiz 49–50, 2006; and Levin B, Lieberman DA, McFarland B, et al: Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology 134(5):1570–1595, 2008.
Adenomatous Polyp and Colorectal Cancer Screening Options
Flexible sigmoidoscopy every 5 years
Double-contrast barium enema every 5 years
CT colonography (CTC, also known as virtual colonoscopy), every 5 years
Colonoscopy every 10 years (should also be performed if any of the above tests are positive)
Most recently, consensus guidelines were released by the American Cancer Society, the American College of Radiology, and the U.S. Multi-Society Task Force on Colorectal Cancer (a group made up of representatives from the American College of Gastroenterology, the American Gastroenterological Association, and the American Society for Gastrointestinal Endoscopy). Computed tomography (CT) colonography is now included as one of several options for colorectal cancer screening every 5 years in average-risk adults, beginning at age 50.6 Moreover, the consensus group now recommends fecal immunochemical testing (FIT) and stool DNA testing (sDNA) as tests that primarily detect cancer.6 In addition to the aforementioned modalities, genetic screening has been applied to patients with a strong history of colorectal cancer.
Over the past two decades, numerous advances have been made in our understanding of the genetic and epigenetic events that lead to colorectal cancers. Although most colorectal tumors (65% to 85%) result from sporadic genetic mutations, 10% to 25% of tumors are considered hereditary. The latter includes about 1% to 3% of patients with hereditary nonpolyposis colon cancer (HNPCC, or Lynch syndrome) and 0.5% with familial adenomatous polyposis (FAP). HNPCC primarily results from germline autosomal dominant gene mutations, truncations, and frameshifts in the DNA mismatch repair genes, MLH1 and MSH2. After the discovery of these two genes, mutations in MSH6 and PMS2 were also linked to HNPCC.7 According to the current InSiGHT (International Society for Gastrointestinal Hereditary Tumors) database maintained by the International Collaborative Group on Hereditary Nonpolyposis Colorectal Cancer (ICG-HNPCC), approximately 500 unique HNPCC-associated mismatch repair gene mutations have been identified. Of these, approximately 50% involve MLH1, approximately 40% involve MSH2, and approximately 10% involve MSH6. In addition, PMS2 mutations are associated with diverse clinical features of HNPCC, including those of Turcot syndrome.
FAP results from germline autosomal dominant mutations in the adenomatous polyposis colic (APC) gene, a tumor suppressor gene, in the Wnt signaling pathway. Inheritance of one or more than 400 known APC mutations carries a 100% lifetime risk of colorectal cancer. In the case of these two hereditary syndromes, as well as with patients with first-degree relative who had colorectal cancer, screening with sigmoidoscopy or colonoscopy as frequently as every 1 to 2 years is a medical necessity. Although these forms of screening have the potential to detect colorectal cancers at early stages, their sensitivity and specificity are not 100%, and there are clearly risks (colonic perforation and hemorrhage) from these invasive procedures. Moreover, compliance with screening is at most 50%.8 Better and noninvasive assays may improve the accuracy, safety, affordability, and patient compliance rates of screening for colorectal cancers.
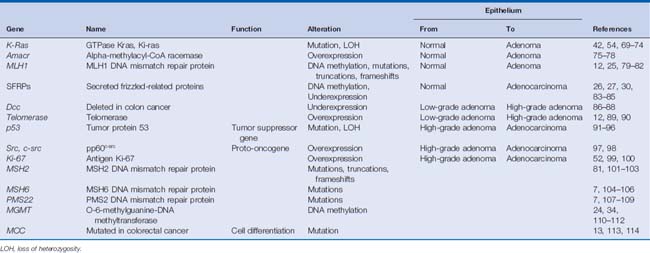
Numerous advances also have been made in identifying genetic factors that are responsible for the progression of sporadic colon cancers. This process is thought to be caused by the accumulation of alterations in DNA, resulting in changes to gene function (i.e., apoptosis resistance, aberrant cellular proliferation, and chromosomal or microsatellite instability), gene mutation or loss of heterozygosity, epigenetic alterations such as DNA methylation and histone changes, as well as over- and underexpression of oncogenes and tumor suppressor genes, respectively.9–11 These genetic and epigenetic alterations lead to the gradual progression of colorectal cancer starting from normal colonic mucosa to metastatic disease through the stages of hyperplastic epithelium, low-grade dysplastic adenomas, high-grade dysplastic adenomas, adenocarcinoma in situ, and invasive adenocarcinoma.12,13 Numerous genes have been identified in this process, and many of the key genes are listed in Table 9-1.
In recent years, researchers have not only used this knowledge to develop new chemotherapeutic agents to treat cancers but also have started to evaluate the usefulness of studying these genes as markers of colorectal tumors with the aim of improving screening outcomes. Many current blood tumor markers, such as carcinoembryonic antigen (CEA), rely on the release of specific proteins into plasma, but these markers have not proved to be useful screening tools but rather are markers for disease recurrence or progression.14
Screening for genetic mutations via stool or blood specimens appears to have several advantages over current FOBT and endoscopic screening methods, as outlined in Box 9-2. The most obvious advantages include a clearer biologic rationale, potentially higher sensitivities for detecting disease at earlier stages, and less reliance on endoscopist experience/subjectivity for evaluating colorectal lesions. Alternatively, these screening methods are expensive and labor-intensive and require additional studies to confirm their validity. In the following sections, we detail some of the research in this growing field.
Box 9-2 Advantages and Disadvantages of Blood/Stool Screening for Colorectal Cancer Gene Mutations
Data from Ahlquist DA: Molecular stool screening for colorectal cancer. Using DNA markers may be beneficial, but large scale evaluation is needed. Br Med J 321(7256):254–255, 2000.
| Advantages | Disadvantages |
|---|---|
Screening Blood
To begin developing a potential bloodscreening method for sporadic colorectal cancers, one group has studied frequent APC and p53 gene mutations in the plasma of 240 colonoscopy patients with colorectal cancer or adenomas.15 In their study, three plasma p53 mutations were identified. Two patients in the study had adenomas at biopsy, whereas one had a hyperplastic polyp. Despite identifying p53 mutations in the plasma, only one (33%) of the adenomas/polyps was positive for the mutation. In the study, only eight tumor specimens were analyzed for concordance with plasma results. One of eight (12.5%) tumors had a 5-base-pair APC gene deletion in the cancer, which was also detectable in that patient’s plasma. Based on this small study, APC gene mutations were detectable in the plasma of a colorectal cancer patient, whereas p53 mutations were detectable in the plasma of adenoma patients. Despite these facts, at present limited data suggest that mutational screening of blood is reliable enough for widespread screening.
Proteomics and nuclear matrix proteins in the blood have also been explored. These limited studies require significantly more evaluation before potential clinical application can be considered.8 Based on a lack of clear evidence, the 2006 American Society of Clinical Oncology recommendation for the use of blood tumor marker tests in the screening and surveillance of colorectal cancers states that data are insufficient to recommend the routine analysis of p53, K-ras, microsatellite instability, deleted in colon cancer (DCC), or other genes in the management of patients with colorectal cancer.14 Current recommendations stipulate only that CEA be ordered preoperatively if it is to assist in staging and surgical planning, whereas postoperative CEA levels should be monitored every 3 months for at least 3 years for patients with stages II and III disease. The CEA blood marker may be used as a marker for monitoring the disease response, but there is no evidence from large study populations to suggest that malignant and premalignant DNA alterations in the colon are identifiable in the blood and may be used for the management of patients.16
Screening Stool
Unlike screening blood for inherited genetic mutations that predispose or lead to cancer, screening stool does not rely on heritability. Instead, it is based on the evaluation of stool specimens for exfoliated DNA from tumors. Because most colorectal cancers are sporadic, they may contain a multitude of genetic and epigenetic abnormalities acquired during the progression of normal mucosa to adenocarcinoma. Since these genetic, as well as epigenetic, alterations to the tumor DNA may not be found in the germline DNA of these patients, screening stool for such alterations appears compelling for identifying cancers. Table 9-2 outlines the biologic rationale for stool screening for colorectal cancer gene mutations and epigenetic alterations.
Table 9-2 Biologic Rationale for Stool Screening for Colorectal Cancer Gene Mutations and Epigenetic Alterations
| Rationale | Note |
|---|---|
| Tumor DNA is continuously released into the fecal stream via exfoliation | As opposed to intermittent bleeding detected by FOBT, this could enhance screening sensitivity, obviating the need for multiple FOBT during each screening |
| DNA is derived directly from the neoplasm | May improve specificity |
| Colonic cell exfoliation from cancers is quantitatively higher than from normal mucosa116,117 | |
| Genetic alterations in colorectal neoplasia are targets for assay development118 | See Table 9-1. |
| DNA is stable during fecal transit and storage | In contrast, RNA is unstable and easily degradable which limits the ability to study the dysregulation of gene expression |
| Bowel preparation and its associated complications, including noncompliance and dehydration, would probably be unnecessary | |
| Sensitive techniques including polymerase chain reaction require only minute quantities of DNA for detection of mutations |
FOBT, fecal occult blood test.
Data from references 115 through 118.
Despite the logic of this approach, targeting genetic alterations such as mutations for widespread screening is not sensitive enough for detecting cancer because no universal mutation and/or epigenetic alteration is common to all colorectal tumors. Rather, they are genetically heterogeneous, as seen in Table 9-1. For example, mutant K-ras is detected in less than 50% of colorectal neoplasms.17,18 Thus, multiple DNA alterations must be selected as targets to obtain a high rate of detection. Furthermore, each marker within the assay must be specific enough to the neoplasm to prevent false-positive results.
Data from pilot projects suggest that the diagnostic yield improves when a stool assay with multiple targets is directed at a spectrum of DNA alterations commonly expressed by cancers.19–21 In a blinded pilot study by Ahlquist and colleagues19 published in 2000, they investigated 15 mutational “hot spots” on genes, including K-ras, p53, APC, and Bat-26.19 DNA alterations were detected in 91% of patients (n = 22) with colorectal cancers, 82% of patients (n = 11) with adenomas larger than 1 cm, and 7% of controls (n = 28) who had had normal colonoscopies. (See Table 9-3 for selected series.).
Table 9-3 Sensitivity and Specificity of Selected Studies Using Stool DNA for Screening of Colorectal Adenomas and Cancers
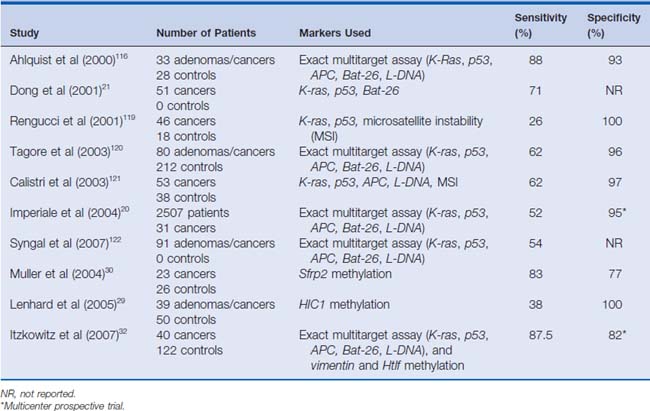
A subsequent study by Dong and colleagues21 in 2001 evaluated stool DNA isolated from 51 patients with confirmed colorectal tumors. Three of the target genes previously evaluated were studied. These included K-ras, p53, and Bat-26. In this study, p53 gene mutations were detected in the tumor DNA of 59% of patients. Tumors from 5.9% of patients had noninherited deletions at the Bat-26 locus. The same alterations were also found in the respective patients’ stool samples. Thirty-seven percent of the tumors had K-ras mutations in their tissue. Among the patients with K-ras mutations, 42% (8 of 19) had the identical mutation seen in the paired-stool DNA. Attesting to the specificity of the assays, no stool specimens contained DNA mutations not found in the primary tumors. Together, these markers detected 71% (36 of 51; 95% confidence interval [CI]: 56% to 83%) of all patients with colorectal cancers. In addition, the markers identified 36 of all 39 patients who had one of the genetic alterations with an overall sensitivity of 92% (95% CI: 79% to 98%). Both of these studies focused on evaluating patients with known disease. Given that the specificity of assays for detecting colorectal neoplasia in asymptomatic patients is unknown and the true prevalence of the mutations is uncertain, there is clearly a need for more studies to evaluate the usefulness of these tests in the general population.
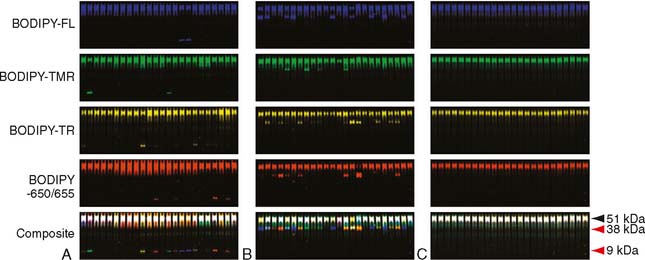
In 2004, Imperiale and colleagues20 studied fecal DNA analysis compared with FOBT for colorectal cancer screening in the average-risk population over 50 years of age. In this study of 5486 subjects, stool specimens were obtained for DNA analysis, and patients underwent both FOBT and colonoscopy. Ultimately, 4404 subjects completed the study, and a subgroup of 2507 subjects was analyzed. This subgroup included all patients diagnosed with a colorectal tumor or advanced adenoma in addition to randomly selected subjects with minor polyps or without evidence of disease. The fecal DNA panel evaluated specimens for 21 mutations. In this study, the expanded fecal DNA panel detected 51.6% of 31 invasive cancers, whereas FOBT identified 12.9% (P = .003). The DNA panel also detected 40.8% of 71 invasive cancers plus adenomas with high-grade dysplasia, whereas FOBT identified 14.1% (P < .001). Among the 418 subjects with advanced neoplasia, which the authors defined as a tubular adenoma at least 1 cm in diameter, a polyp with a villous histologic appearance, a polyp with high-grade dysplasia, or invasive cancer, the DNA panel was positive in 18.2% of cases, whereas FOBT was positive in 10.8%. The authors concluded that the multi-target analysis of fecal DNA detected a greater proportion of important colorectal neoplasia than did FOBT without compromising specificity. (See Fig. 9-1 for an example.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree