Abstract
The concept of the stem cell of origin for cancers was first proposed more than 100 years ago. In this theoretical model, certain cells with self-renewal capacity would form tumors from “embryonic rests.” More evidence has accumulated since that time as a result of extensive research that strongly supports the cancer stem cell (CSC) hypothesis , suggesting the existence of self-renewing cells that generate heterogenous populations of cells within the tumor mass. The preponderance of evidence now suggests that the majority of cancers are hierarchically organized and sustained by a population of cells that display stem cell properties—CSCs. As is the case for normal tissue stem cells, CSCs are able to self-renew and differentiate, generating cells that comprise the tumor bulk. Furthermore, preclinical and clinical studies demonstrate that CSCs mediate tumor metastasis and contribute to resistance to chemotherapy and radiation therapy. The CSC hypothesis has fundamental biological and clinical implications that are discussed in detail in this chapter.
Keywords
stem cell, cancer stem cell, CSC, tumor initiating cell, stem cell immunotherapy
The concept of the stem cell of origin for cancers was first proposed more than 100 years ago. In this theoretical model, certain cells with self-renewal capacity would form tumors from “embryonic rests.” More evidence has accumulated since that time as a result of extensive research that strongly supports the cancer stem cell (CSC) hypothesis , suggesting the existence of self-renewing cells that generate heterogenous populations of cells within the tumor mass. The preponderance of evidence now suggests that the majority of cancers are hierarchically organized and sustained by a population of cells that display stem cell properties—CSCs. As the case for normal tissue stem cells, CSCs are able to self-renew and differentiate, generating cells that comprise the tumor bulk. Furthermore, preclinical and clinical studies demonstrate that CSCs mediate tumor metastasis and contribute to resistance to chemotherapy and radiation therapy. The CSC hypothesis has fundamental biological and clinical implications that are discussed in detail in this chapter.
Stem Cells in the Normal Breast
Embryologic studies of the normal mammary gland indicate that stem cells in the mammary bud develop into early progenitors and then late progenitors, which then differentiate into either ductal epithelial cells or myoepithelial cells. Ductal and alveolar epithelial cells express MUC1 and epithelial keratins, whereas myoepithelial cells express common acute lymphoblastic leukemia antigen (CALLA) and other keratins including CK14. Ductal and alveolar epithelial cells are fundamentally different from myoepithelial cells. Ductal and alveolar epithelial cells can proliferate, secrete casein, and express hormonal receptors. Myoepithelial cells, in contrast, synthesize a basement membrane, do not express hormone receptors, and undergo only limited proliferation. Both cell lineages are thought to be derived from undifferentiated mammary stem cells. These normal mammary stem cells characterized as CD49f + /EpCAM − are capable of self-renewal and differentiation both in vitro and in vivo. In fact, obligate features of stem cells are their ability both to self-renew and to differentiate. Self-renewal may consist of either symmetric cell division (into two daughter stem cells) or asymmetric division (into one daughter stem cell and one proliferating cell capable of subsequent differentiation). This differentiation may occur across several different lineages, and thus breast stem cells are characterized as multipotent. For example, a normal mammary stem cell can differentiate into either a ductal or alveolar epithelial cell or myoepithelial cell. The ability of mammary stem cells to exhibit evidence of “stemness” can be assayed in two-dimensional culture, three-dimensional culture, and via transplantation into the cleared mammary fat pad of syngenic mice. In in vitro assays, predetermined growth conditions with serum, growth factors, and Matrigel are used. One important in vitro property exhibited by mammary stem cells is the property of forming mammospheres, tight aggregates of cells in suspension culture in serum-free media supplemented with growth factors. Single mammary stem cells also have the ability to exhibit alveolar and ductal morphogenesis when cultured in three-dimensional structures in Matrigel. In the developing normal breast, aldehyde dehydrogenase 1 (ALDH1)-positive stem cells, which are also thought to be estrogen receptor (ER) negative, are thought to generate ER-positive progeny.
Markers of Normal Stem Cells
The normal mammary tissue consists of luminal and mesenchymal stem cells that are responsible for self-renewal and expansion associated with cycles of pregnancy that form mammary structures containing luminal, alveolar, and myoepithelial cells. Studies have demonstrated that mammary stem cells are enriched within a CD49f + /EpCAM − population that is also more closely associated with a basal phenotype. Another marker that is commonly used to define normal mammary stem cells is the enzyme aldehyde dehydrogenase (ALDH). Increased ALDH activity also characterizes hematopoietic and neuronal stem/progenitor cells, where it has been demonstrated to play a functional role in stem cell differentiation. The activity of ALDH can easily be determined by an enzymatic assay termed the Aldefluor assay (Stemcell Technologies, Vancouver, Canada), and a monoclonal antibody detecting the ALDH1 protein can also be used for immunodetection. Studies of normal breast have revealed that approximately 6% of the epithelial cells within the ductal-lobular units were Aldefluor positive. Only ALDH + cells generated mammospheres in suspension culture. In nonobese diabetic mice with severe combined immunodeficiency, only ALDH + cells formed ductal structures. Recent studies have shown that different ALDH isoenzymes characterize different mammary stem cell populations. ALDH1a3 is predominantly expressed in stem cells in alveolar “cap” cells, whereas ALDH1a1-expressing stem cells are predominantly found in mammary ducts at branch points.
Mammary Stem Cell Regulatory Pathways
A number of signaling pathways have been implicated in the regulation of normal stem cell self-renewal, lineage commitment, and differentiation. These signaling pathways are activated via ligand activation of membrane receptors, which signal through cytoplasmic intermediaries ultimately regulating gene transcription. Key stem cell signaling pathways include hedgehog (Hh), Notch, WNT, and AKT pathways. Hedgehog signaling regulates mammary stem cell self-renewal, as demonstrated by an increase in mammosphere formation after hedgehog pathway activation. Furthermore, increased expression of hedgehog pathway ligands and receptors are found in mammospheres compared with two-dimensional cultures. Notch signaling is initiated via binding of Notch receptors to ligands expressed on adjoining cells. This results in Notch activation via gamma secretase mediated cleavage. The resulting Notch intracellular domain (NICD) then translocates to the nucleus where it activates a number of bHLH transcription factors including HES1, HES5, HEY1, and HEY2. This results in increased stem cell self-renewal as measured by mammosphere formation. Hedgehog and Notch pathways may regulate stem cell self-renewal through regulation of the polycomb gene BMI-1 . These signaling pathways do not operate in isolation but are thought to interact with each other as well as other regulatory pathways.
A number of signal transduction pathways may regulate mammary stem cell fate decisions via modulation of microRNAs (miRNAs), which are small, noncoding RNA sequences that can act as potent regulators of stem cell fates. miRNAs have been shown to be involved in several key stem cell regulatory pathways that affect the proliferation and differentiation of normal mammary stem cells. For example, Mir 93 regulates mammary stem cells via modulation of TGFBR2, whereas Mir 200 regulates these cells via modulation of BMI-1 .
Stem Cells in Breast Cancer
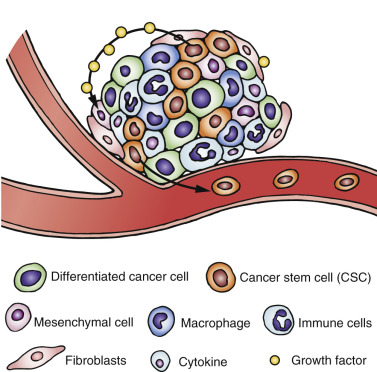
Despite compelling evidence that all cancers, including breast cancer, are monoclonal in origin, established tumors display marked cellular heterogeneity. In fact, this heterogeneity represents the greatest challenge to the development of effective cancer therapeutics. Several models have been proposed to explain this cellular heterogeneity. The stochastic model proposes that cancers develop through a process involving random “stochastic” mutation followed by clonal selection. The term stochastic emphasizes that the mutations that develop are random. Multiple rounds of mutation and clonal selection generate genetically heterogenous tumors through a process resembling darwinian evolution. In this model, all of the tumor cells are equally malignant. The CSC model, in contrast, posits that tumors are hierarchically organized and driven by a subpopulation of cells that display stem cell properties. The stochastic model governed our thinking about cancer in the 1980s, whereas the stem cell model has been gaining in popularity since the mid-2000s. However, it is important to emphasize that these two models of carcinogenesis and tumor heterogeneity are not mutually exclusive, and in fact there is now substantial evidence that elements of both apply to most tumors. In such a “hybrid model,” tumors arise in self-renewing cell populations, but the resulting CSCs are themselves genetically unstable and subject to mutation and clonal selection. As a result, tumors may be composed of genetically diverse CSCs as well as their more differentiated progeny ( Fig. 23.1 ).
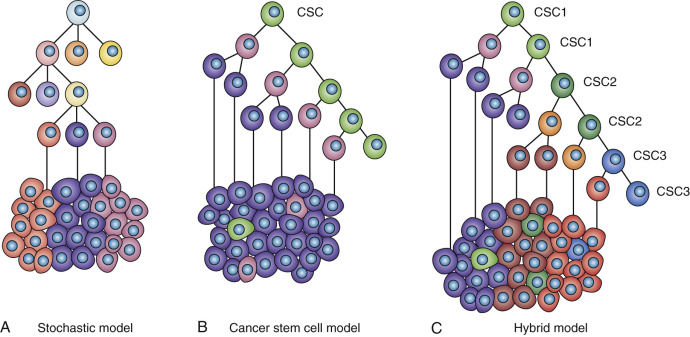
Evidence supporting the stem cell model in breast cancer is based on studies with established human breast cancer cell lines, and more recently in patient-derived xenograft (PDX) models that were sorted on the basis of putative stem cell markers such as CD44 + /CD24 low/− /LIN. Cells expressing the stem cell markers were demonstrated to be more tumorigenic compared with the cells lacking expression of these markers. Similar results were obtained using a different stem cell marker, ALDH, where ALDH + cells were considerably more tumorigenic than ALDH − cells when introduced into immunosuppressed NOD-SCID mice. Some researchers argued, however, that these experiments were biased toward selecting for cells with the capacity to grow in the immunocompromised mouse microenvironment rather than characteristics of stemness per say. It was argued that if one did the same experiment with a murine tumor in a syngeneic murine host, this differential tumorigenicity might not be observed. However, a number of studies using genetically engineered mouse mammary tumor models have demonstrated that these tumors contain subpopulations of CSCs capable of generating tumors when transplanted into immunocompetent syngeneic mice. Although these immune competent mice may represent a more physiologically relevant microenvironment, these studies are still open to the criticism in that serial transplantation of tumor cells disrupts the microenvironment in which these tumors naturally develop. However, three recent landmark studies addressed these arguments by using lineage tracing to demonstrate in three tumor types that tumors originate in self-renewing stem cell populations generating tumors containing CSCs. The relevance of these findings to human cancers was further demonstrated using NextGen (Illumina, San Diego, CA) sequencing of CSC populations in human leukemia demonstrating signatures common to normal hematopoietic stem cells.
Markers of Cancer Stem Cells
As noted previously, CSCs display a number of markers that are shared by normal stem cells. This includes CD44 + /CD24 low/− and ALDH. In addition, embryonal stem cell markers often expressed by CSCs include stellar, rex-1, nestin, and H19, as well as transcriptional factors such as nuclear β-catenin. In addition, OCT4, NANOG, and SOX2 the core transcription factors involved in iPS stem cell reprogramming, are also expressed in a number of CSC populations. This raises the interesting possibility that activation of these factors reprograms more differentiated tumor cells into a stemlike state during carcinogenesis. An ever-growing list of cancer stem markers is being described and characterized ( Tables 23.1–23.3 ). One stem cell marker is the ATP-binding cassette transporter protein ABCG2, which facilitates efflux of lipophilic dyes such as Hoechst 33342, identifying a population of cells that is termed the side population on flow cytometric analysis. This transporter is also thought to efflux chemotherapeutic drugs from CSCs rendering them resistant to chemotherapy.
| Stem Cell Type | Molecular Marker | Significance |
|---|---|---|
| Embryonic stem cells (ES) or pluripotent stem cells (PS) | Oct-4 | Transcription factor essential for establishment and maintenance of undifferentiated PS |
| Pax-6 | Transcription factor expressed as ES differentiates into neuroepithelium | |
| Stellar | Specific marker of undifferentiated ES | |
| Alpha-fetoprotein (AFP) | Reflects endodermal differentiation of PS | |
| Rex-1 | Specific marker of undifferentiated ES | |
| Germ cell nuclear factor (GCNF) | Transcription factor expressed by PS | |
| Sox-2 | Transcription factor essential for establishment and maintenance of undifferentiated PS | |
| H19 | Marker developmentally regulated in skeletal muscle, smooth muscle, and fetal liver | |
| Nanog | Transcription factor unique to PS; essential for establishment and maintenance of undifferentiated PS | |
| Hematopoietic stem cells (HS) | CD34 | Indicative of HS and EP |
| c-kit | Cell surface receptor on bone marrow cell types that identifies HS and MS | |
| Stem cell antigen (Sca-1) | Indicative of HS and MS in bone marrow and blood | |
| Mesenchymal stem and progenitor cells (MS) | Bone morphogenetic protein receptor (BMPR) | BMPR identifies early mesenchymal lineages (MS) |
| Stro-1 antigen | Cell surface glycoprotein on subsets of bone marrow MS | |
| Neural stem cells (NS) | CD133 | Identifies NS and HS |
| Nestin | Identifies NS | |
| Endothelial progenitor cells (EP) | Fetal liver kinase-1 (Flk-1) | Cell surface receptor protein that identifies EP |
| Human Cancer Type | Phenotypic Marker | Side Population |
|---|---|---|
| Leukemia | CD34 + /CD38 − , CD44 + | Yes |
| Breast cancer | CD44 + /CD24 low/− , ALDH + , mammosphere formation | Yes |
| Prostate cancer | CD44 + /α 2 β 1 hi /CD133 + , Sca-1 + | Yes |
| Melanoma | CD20 + , spheroid formation | Yes |
| Brain cancer | CD133 + , neurospheroid formation | Yes |
| Retinoblastoma | ABCG2 + , ALDH1 positive | Yes |
| Colon cancer | CD133 + | Yes |
| CD44 + /CD133 + /CD24 low/− , spheroid formation | No |
| Stem Cell Properties Exhibited in Vitro | |
|---|---|
| Stem cell–specific markers | Transcriptional determinants as well as specific markers (e.g., oct-4, sox-2, nanog, rex-1) known to be restricted to normal embryonal or tissue stem cells detected within cancer stem cells |
| Self-renewal and proliferation | Ability of single cancer stem cell to generate secondary spheroids (mammospheres) without initial cell aggregation/cell-cell contact; spheroids containing as few as 100 cells are fully tumorigenic. |
| Multipotency | Ability of single cancer stem cell cultured in Matrigel to manifest multipotency or differentiation |
Key Signaling Pathways of Cancer Stem Cells
The same signaling pathways that regulate normal stem cells are thought to play similar roles in CSCs. However, in CSCs, these pathways may be constitutively activated through genetic or epigenetic events. Developmental pathways that have been demonstrated to play an important role in CSC function include Hh, Notch, WNT, and Akt pathways. Evidence for the importance of these pathways in CSCs has been derived from experiments showing that pathway activation increases and pathway inhibition reduces the proportion of CSCs. The similarities between signaling pathways in normal and malignant stem cells presents a potential challenge for the development of CSC targeted therapeutics.
Many of the genes implicated in breast carcinogenesis including BRCA1 , HER2 , and PTEN have also been demonstrated to play important roles in mammary stem cell self-renewal and differentiation. This supports the hypothesis that breast cancers originate in self-renewing cell populations through aberrant activation of these pathways. BRCA1 is a gene in which a germline mutation can confer an 80% risk of breast cancer. In addition, the majority of breast cancers occurring in BRCA1 cancers are “triple negative,” with significant expression of basal markers (CK5 and CK6). In addition to hereditary breast cancers, a subset of sporadic triple-negative breast cancers (TNBCs) may result from inhibition of somatic BRCA1 expression via gene methylation. BRCA1 plays an important role in mammary stem cell differentiation and downregulation of BRCA1 in normal breast cells increases the proportion of stem cells as evidenced by increased mammosphere formation and ALDH expression. In women with germline BRCA1 mutations, expansion of ALDH-expressing cells in mammary lobules is associated with loss of heterozygosity of the normal allele. Together these studies demonstrate that in addition to its well-known role in DNA repair, BRCA1 ‘s role in mammary carcinogenesis may also relate to its importance in mammary stem cell regulation.
HER2/neu is amplified in 20% of breast cancers and is associated with a more aggressive clinical course. The development of HER2 targeted therapies for the treatment of HER2-positive tumors represents one of the most significant advances in clinical oncology. The clinical efficacy of these agents may relate to the role that HER2 plays in the regulation of breast CSCs. In vitro and in mouse models, HER2 overexpression increases the CSC population, and trastuzumab targets and reduces this population. More recently we have reported that in luminal breast cancers, HER2 may be selectively expressed in CSCs in the absence of HER2 gene amplification. This might account for the surprising finding that HER2 blockade in the adjuvant setting might extend to women whose breast tumors do not display HER2 gene amplification. In addition, this might account for the report of detection of HER2-expressing cells in the blood circulation of women with HER2-“negative” breast cancers. The most frequent genetic alteration associated with trastuzumab resistance is PTEN deletion. We have recently demonstrated that PTEN deletion activates an inflammatory loop mediated by the cytokine interleukin (IL)-6. This suggests novel strategies to target trastuzumab resistant breast cancer via IL-6 blockade.
Relationship of Epithelial-Mesenchymal Transition and Cancer Stem Cell States
A number of studies have suggested similarities between the CSC phenotype and acquisition of an epithelial-mesenchymal transition (EMT) state. The EMT is a developmental process that occurs during embryogenesis and tissue formation, which involves loss of epithelial and acquisition of mesenchymal characteristics of migrating cell populations. EMT is a reversible process and through the reverse process mesenchymal-epithelial transition (MET) mesenchymal cells may reacquire an epithelial phenotype. There is evidence that tumor cells may undergo both EMT and MET during the process of metastasis. A number of transcription factors, including SNAIL, SLUG, and TWIST have been reported to be involved in EMT. It has been shown that breast CSCs express a number of genes involved in EMT, and conversely breast cancer cells that undergo EMT become stemlike. Researchers have demonstrated that conditions that induce EMT in human breast cancers, such as hypoxia or addition of transforming growth factor beta (TGFβ), also increase the proportion of cells expressing the CSC phenotype CD44 + /CD24 low/− . We have recently demonstrated that in human breast cancer, CSCs exist in alternate states that are characterized by expression of different markers and properties. EMT-like CSCs that have been characterized as CD44 + /CD24 low/− are highly invasive but relatively quiescent. In contrast, the more epithelial or “MET”-like CSCs, which are characterized by ALDH expression, are more proliferative and capable of “self-renewal.” Furthermore, CSCs display plasticity, being able to transition between EMT-like and MET-like states in a process regulated by the tumor microenvironment. As determined by immunohistochemistry, the EMT-like CD44 + /CD24 low/− CSCs are primarily found at the tumor invasive front, whereas MET-like ALDH + CSCs are primarily located more centrally. This suggests a model in which EMT CSCs at the tumor invasive front enter the circulation, where they travel to generate metastases at distant sites. These EMT-like micrometastases are nonproliferative and remain dormant until they are induced to convert to an MET “self-renewing” state in which they generate additional CSCs as well as the more differentiated cells that form the tumor bulk. This model is supported by studies demonstrating that both circulating tumor cells as well as disseminated micrometastatic cells in the bone marrow of breast cancer patients are enriched in nonproliferative (KI67 neg) CD44 + /CD24 low/− cells. In contrast, both primary tumors and macrometastases contain both CD44 + /CD24 low/− and ALDH + CSCs.
Cancer Stem Cells and the Tumor Microenvironment: Clinical Implications
Cancer Stem Cells and the Immune System
The complexities of CSC biology outlined thus far in this chapter illustrate the potential, unique challenges for the design of effective therapies to combat tumor development and breast cancer reoccurrence. Indeed, the tumor microenvironment presents a formidable ecological niche that was once considered insurmountable. The immune responses and alterations that occur within this microenvironment involve an intricate network of signaling pathways, immune cell interactions, mediators, cytokine loops, and epigenetic alterations as immune cells interplay between tumor cells and the stroma ( Fig. 23.2 ). To further complicate the process, all of these events occur against the backdrop of dynamic EMT-MET transitions associated with the CSCs. However, it is this very diversity, and the dynamic nature of this process, that also presents a multitude of therapeutic opportunities ( Box 23.1 ). Furthermore, the elegant balance between the hosts’ innate immune activation and suppression, as well as commonalities of immune regulation shared between various disease states, could potentially be leveraged to elicit immune responses aimed not only at reducing the tumor bulk but also at targeting and destroying the cancer stem populations responsible for tumor metastasis.