Fig. 1
Platinum complexes which entered clinical trials
While most of the platinum drugs have to be administered intravenously, Satraplatin (8) was the first orally applicable platinum agent under clinical investigation (Fig. 1). Like iproplatin it is a Pt(IV) complex prodrug which is reduced in the organism to active Pt(II) complexes (activation by reduction) that build up DNA lesions similar to cisplatin [24]. Satraplatin when given alone rendered a partial response rate of 38 % in patients with SCLC. Clinical trials in combination with paclitaxel for patients with NSCLC are ongoing [25]. Picoplatin (9) is another orally applicable Pt(II) complex initially designed to meet the glutathione-mediated resistance towards platinum drugs (Fig. 1). The methyl group of the picoline ligand hinders the attack of deactivating bionucleophiles such as glutathione and metallothionein at the Pt(II) center, and an enhanced activity of picoplatin in resistant cancer cells was actually observed [26, 27].
Ruthenium Complexes
While primary solid tumors can be efficiently removed by surgery in many cases the emergence of metastases is frequently responsible for the lethal issue of cancer diseases. Hence, suitable treatments which prevent or reduce metastasis are in great demand. The promising ruthenium(III) based drug candidate NAMI-A (“New Antitumor Metastasis Inhibitor”), ImH[trans-Im(DMSO)RuCl4] (Fig. 2) initially failed the common anticancer screens such as the NCI 60 cell lines screen yet prevented in additional animal studies the development and growth of lung metastases derived from lung and breast carcinomas [28–30]. The high efficacy of NAMI-A in the lungs is based on its eightfold longer half-life in lung tissue compared with other tissues and organs which is probably due to the high content of collagen which can interact with NAMI-A. In addition to its anti-invasive properties NAMI-A also possesses anti-angiogenic activity as to assays with blood vessels in the chorioallanthoic membrane (CAM) of chicken and in rabbit cornea probably by binding to NO released from the endothelial cells [31]. In contrast to platinum drugs NAMI-A binds only weakly to DNA. NAMI-A inhibits the MAPK pathway by suppression of membrane PKC leading to apoptosis and it reduces cell migration as well as the release of gelatinase [32, 33]. A phase I study in 2004 revealed a high tolerance of the drug by patients with the appearance of blisters being the only dose-limiting toxic event [34].
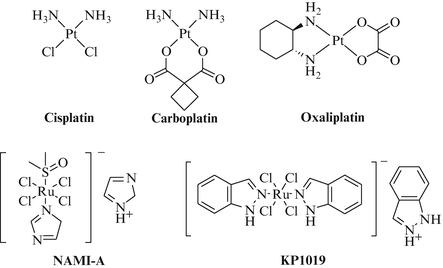

Fig. 2
Platinum and ruthenium complexes with proven anticancer activity
Meanwhile NAMI-A type compounds with improved stability in aqueous solutions have emerged. Complex 10 (Fig. 3), for example, with a less basic pyrazole ligand distinctly reduced the number of lung metastases arising from Lewis lung carcinoma and MCa breast carcinoma [35]. In the lung cancer model 10 showed similar activity compared with NAMI-A while in the breast cancer model it was twice as active as NAMI-A with respect to the reduction of lung metastases. Thus, complex 10 behaves similarly to NAMI-A. However, it did not arrest the cell cycle at the G2/M checkpoint like NAMI-A. On the other hand, 10 inhibited tumor cell migration in matrigel more efficiently than NAMI-A. Another NAMI-like complex is (Hdmtp)[trans-RuCl4(DMSO)(dmtp)] (11) bearing a 5,7-dimethyl[1, 2, 4]triazolo[1,5-a]pyrimidine ligand (dmtp) (Fig. 3) [36]. Complex 11 exhibited antimetastatic properties comparable to those of NAMI-A, but also revealed a higher liver toxicity and caused edema. However, it showed a lower kidney toxicity than NAMI-A.
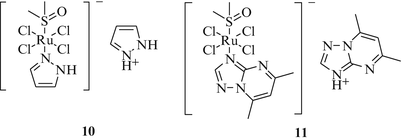

Fig. 3
NAMI-A-type Ru(III) complexes with high activity against lung cancer and lung metastases in lab animals
Organoruthenium complexes are usually more stable than NAMI-A-type coordination complexes concerning ligand exchange and were therefore identified as promising candidates for new anticancer drugs as well. RAPTA-C (12) is a ruthenium(II)-arene complex bearing a 1,3,5-triaza-7-phosphaadamantane (PTA) ligand (Fig. 4). The η6-arene ligand (p-cymene) stabilizes the reactive Ru(II) state of the complex. Complex 12 showed pH dependent DNA damage, in particular at lower pH values which are typical of hypoxic tumor sites when the PTA ligand is protonated [37]. Despite of the structural differences between NAMI-A and RAPTA-C (12) their modes of action showed similarities. Like NAMI-A complex 12 is inactive against the primary tumor, yet highly efficacious against lung metastases. Treatment of MCa mammary carcinoma bearing CBA mice with 200 mg/kg/day of 12 for 2 days reduced the number of lung metastases by more than 50 % with two mice of the treated group turning entirely cancer free [38]. While the half-life of complex 12 is comparable with that of NAMI-A it showed enhanced distribution and blood clearance rates. Hence, complex 12 (RAPTA-C) appears a suitable candidate for further testing including clinical trials.
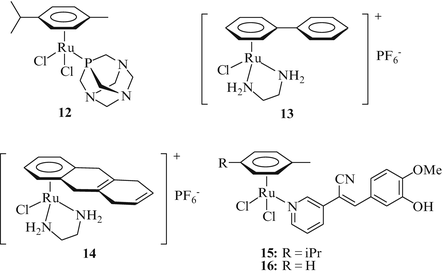

Fig. 4
Organometallic arene-Ru(II) complexes with potential against lung cancer
Sadler and co-workers prepared the ruthenium(II) complexes RM175 (13) and HC11 (14) which exhibit significant cytotoxic activity in cancer cells, including cisplatin-resistant cells (Fig. 4) [39]. These complexes coordinate selectively to the N7-site of guanine bases forming an additional hydrogen bond between an NH2 group of the en ligand and the C6-carbonyl group of guanine. Both complexes were distinctly growth inhibitory in A549 lung non-small cell lung cancer xenografts at doses of 25 mg/kg. A significant liver toxicity was observed at higher doses for complex 14. The authors suggest that liver toxicity is attributed to the arene ligand and further modification of the arene ligand is in progress in order to reduce the hepatotoxicity of such ruthenium complexes. Inspired by the successful launch of the EGFR-inhibitor erlotinib as a drug against advanced NSCLC, Biersack et al. have recently prepared neutral arene-Ru(II) complexes 15 and 16 of pyridine-based tyrphostine derivatives which are well known EGFR inhibitors (Fig. 4) [40, 41]. These complexes exhibited growth inhibitory activity in various cancer cell lines at sub-micromolar concentrations and led to strong DNA metallation in vitro.
Motexafin Gadolinium
Between 100,000 and 300,000 cancer patients are diagnosed with brain metastases in the US alone every year and their survival expectancy is dauntingly poor [42]. Since radiation therapy is the treatment of choice for brain metastases, radiosensitizing agents were developed in order to improve the impact of the radiation therapy and to reduce side effects. The MRI-detectable complex motexafin gadolinium (17, MGd), also known as gadolinium texaphyrin (Xcytrin, NSC 695238) is a metal-based anticancer agent that showed promising results as a radiosensitizer (Fig. 5). This agent features a Gd(III) ion chelated by a porphyrin-like texaphyrin ligand. Due to its high electron affinity complex 17 readily oxidises cellular components, e.g., reducing metabolites, leading to lethal DNA damage [43]. In addition, complex 17 directly targets enzymes like thioredoxin reductase and ribonucleotide reductase which are often overexpressed in cancer cells [44], and it interferes with intracellular zinc levels in cancer cells probably due to a direct oxidation of zinc metallothionein [45]. The pleiotropic anticancer effects of 17 led to various clinical trials, e.g., against NSCLC and brain metastases thereof. In these trials the performance of complex 17 is characterised by a high tumor-selectivity and tolerable side-effects and toxicities. In combination with whole-brain radiation therapy (WBRT) complex 17 revealed reduced neurotoxicities and neurological progression as well as improved quality of life in patients with NSCLC-derived brain metastases in a phase III study (SMART) [46].
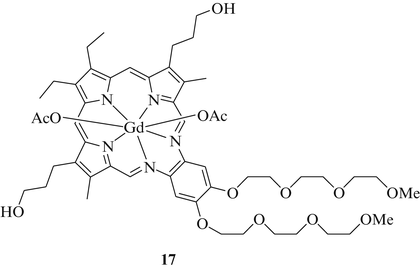

Fig. 5
Motexafin gadolinium (17)
Ferrocene-Based Compounds
The discovery of ferrocene (18, Fig. 6) and the elucidation of its sandwich structure in the 1950s by G. Wilkinson (Imperial College London) and E. O. Fischer (Technical University Munich) was a milestone in the field of inorganic chemistry and was awarded with the Nobel Prize in 1973. The simple complex ferrocene activates lymphocytes and exerts distinct antitumor effects by redox-sensitive signaling involving oxidation of Ras proteins at cystein sites [47]. Recently, Kenny and coworkers prepared ferrocene conjugates bearing peptide fragments. Complex 19, for instance, features a combination of a ferrocene component, a naphthoyl linker and a small peptide moiety which has shown excellent activity in the H1299 non-small cell lung cancer cell line (IC50 = 0.62 μM) and so exceeded the efficacy of cisplatin in these tumor cells by far (Fig. 6) [48, 49]. The naphthoyl linker of complex 19 lowers the oxidation potential of the conjugated ferrocene while the peptide fragment may form many hydrogen bonds to the biological targets. Snegur and coworkers prepared the new ferrocenylethyl benzotriazole 20 and studied it concerning its in vivo activity in lung cancer xenografts via the subrenal capsular assay (SCA) in comparison with cisplatin (Fig. 6) [50]. Complex 20 exhibited low toxicity and caused 45 % regression in non-small cell lung cancer models at doses of 18 mg/kg/day, while cisplatin led to merely 23 % regression in this tumor model. The same group found that complex 20 alkylates nucleobases like adenine forming ferrocenylalkyl adenine. This mild way of ferrocenylalkylation is assumed to be the reason for the selective triggering of endonucleases at the early stage of apoptosis without causing necrotic effects. In continuation of this interesting discovery Simenel et al. have recently disclosed new ferrocene conjugates with nucleobases. Ferrocenylmethyl thymine 21 showed distinct in vivo activity in various solid tumors (Fig. 6) [51]. For instance, mice bearing Lewis lung carcinoma (LLC) when treated with 21 (5.0 mg/kg/day intraperitoneally) revealed a growth inhibition of the LLC tumor of 45 %. The combination of complex 21 (5.0 mg/kg/day) with the alkylating anticancer drug cyclophosphamide led to improved effects in the LLC xenografts (growth inhibition of 50 %). The maximum tolerated dose (MTD) for 21 was 20 mg/kg when given intraperitoneally. The same group also reported the preparation of a ferrocenylalkyl thiopyrimidine 22 (Fig. 6) with enhanced anticancer activity that can be given to mice at a higher dose than 21 [52]. LLC xenografts were treated with 22 (20 mg/kg/day) and showed a tumor growth inhibition of 65 %. The observed MTD for complex 22 was 150 mg/kg.
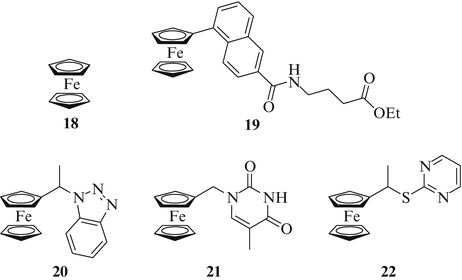

Fig. 6
Ferrocene-based compounds with activity against lung cancer
The compound class of retinoids comprises both natural vitamin A derivatives and synthetic analogs which are important for the differentiation of cells and the development of tissues and organs [53–55]. These effects are mediated by the binding of retinoids to nuclear receptors such as retinoic acid receptors (RARs) or retinoid X receptors (RXRs). All-trans-retinoic acid (ATRA) and bexarotene, for example, are currently applied for the treatment of various skin and cancer diseases. Their use is limited, though, by teratogenic and hepatotoxic side-effects [56–58]. Xiang and coworkers prepared new 13-cis-retinoyl ferrocenes in order to enhance the biological activity of retinoids against cancer [59]. Compound 23 (Fig. 7) exhibited improved activity in A549 lung cancer cells (IC50 = 20.4 μM) compared with 13-cis-retinoic acid (IC50 = 35.8 μM) alone. Jaouen and coworkers disclosed several ferrocene derivatives (so-called ferrocifens) of the selective estrogen receptor modulator (SERM) tamoxifen [60, 61]. A compound dubbed hydroxyferrocifen exhibited distinct growth inhibitory activity in breast cancer cells, both ER-positive and ER-negative. Recently, Marques et al. disclosed a ferrocene-based raloxifen analogue 24 which also displayed some activity in lung cancer cells (Fig. 7) [62]. In A549 lung cancer cells complex 24 exhibited a distinctly better growth inhibition (IC50 = 3.85 μM) than cisplatin (IC50 = 18.13 μM). Generally, compound 24 showed no cross-resistance to cisplatin and is obviously no substrate for the ABC-transporters of multi-drug resistant cells.
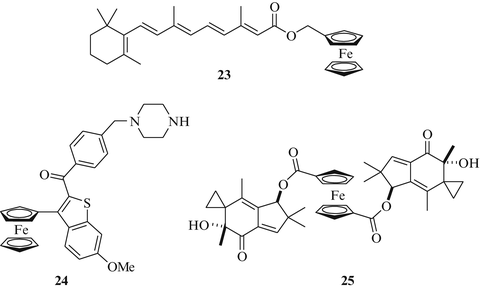

Fig. 7
Ferrocene conjugates with anticancer active compounds
Ferrocenes can also increase the selectivity of anticancer agents and shield reactive drugs sterically or electronically as observed in the case of the fungal cytotoxin illudin M. Illudins M and S and the semisynthetic compound irofulven are alkylating agents which are activated by reduction of their enone system either by NADPH-dependent oxido-reductases or by glutathione [63]. Irofulven was found to be efficacious against MV522 metastatic lung carcinoma xenografts and to increase the life expectancy of treated animals by more than 150 %, a value that exceeds the one of mitomycin C by far (increase in life span of 61 %) [64]. In addition, irofulven blocked the formation of lung metastases in an animal model resistant to classical anticancer drugs. However, irofulven remained inactive in a clinical phase II trial against advanced non-small cell lung cancer [65]. In a search for more selective illudin M derivatives, Schobert and coworkers developed ferrocene conjugates of illudin M [66, 67]. The ferrocene diester 25 (Fig. 7) was active at sub-micromolar IC50 values against various cancer cell lines while being forty times less toxic than illudin M in non-malignant fibroblast cells. The ferrocene-attached illudin M moieties of 25 are less prone to reductive detoxification by glutathione than free illudin M, and its antiproliferative activity depends on active JNK-signaling. First in vivo assays of 25 in lab mice revealed no toxicity at doses of 25 mg/kg and further tests in suitable lung cancer xenografts are planned.
Conclusions
The platinum complexes cisplatin and carboplatin have been a mainstay in the therapy of lung cancer diseases right from the beginning. More platinum complexes were put through their paces in clinical trials and a good deal of them was found active against drug resistant and advanced lung cancers. In addition to platinum compounds, ruthenium and gadolinium complexes have emerged that selectively target metastases in the lung or lung cancer metastases in the brain. The drug candidates NAMI-A and motexafin gadolinium have already reached advanced stages of clinical trials and are likely to obtain approval for the treatment of lung cancer metastases. Organometallic sandwich complexes such as ferrocene pose a second line of development in the field of anticancer metallodrugs. When attached to intrinsically bioactive fragments complex conjugates with pronounced activity in lung cancer models may result. Ferrocenes in particular figure prominently among such conjugates, presumably due to their interference with reactive oxygen species and with detoxification of drugs.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree