Fig. 1
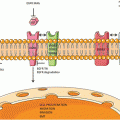
Vascular endothelial growth factor receptor signal transduction (Reprinted with permission from Roskoski. CROH 2007 62(3):179–213)
The role of serum VEGF (VEGF-A) in tumor growth and development has been widely studied. Serum VEGF activates the VEGF receptor (VEGFR-2) resulting in downstream signalling through activation of targets including PI3-kinase, resulting in stimulation of cell survival and proliferation pathways [7, 8]. Overexpression of VEGF-A appears to be a negative prognostic factor for survival [9]. Given its role in tumor progression and negative impact on survival, the VEGF/VEGF-R pathway has been the focus of extensive therapeutic evaluation for anti-angiogenic agents.
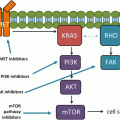
The Platelet Derived Growth Factor (PDGF) family of receptors represent an additional pro-angiogenic pathway. PDGF results in stimulation of PDGF receptor α (PDGFR-α) and PDGF receptor β (PDGFR-β) [4, 10] resulting in downstream signalling through PI3K and extracellular signal-regulated kinase (Erk) [11]. In vitro data suggest that PDGFR expression in tumors is a mechanism of resistance to VEGF directed therapy and provide a rationale for combined VEGF and PDGF directed therapy [12, 13].
Fibroblast Growth Factor (FGF) and its receptor represent a further anti-angiogenic target [14]. Similar to VEGF and PDGF, ligand binding causes receptor dimerization, activation of the tyrosine kinase domain, and stimulation of downstream targets, including PI3K and the mitogen-activated protein kinase (Mek)-Erk pathways [5]. In vitro data suggest synergism between FGF, VEGF, and PDGF pathways in stimulating angiogenesis and cellular growth [15, 16]. Resistance to anti-VEGF therapy may be due in part to upregulation of compensatory angiogenic signaling pathways (cross-talk), such as PDGF and FGF, therefore, inhibition of multiple pro-angiogenic pathways may represent a rational treatment strategy for patients with NSCLC.
These data provide a rationale for the evaluation of agents inhibiting VEGF, PDGF, or FGF as treatment options for lung cancer. Anti-angiogenic agents have been widely evaluated in combination with standard systemic therapies. There are several proposed mechanisms of action through which anti-angiogenic therapies can provide further benefit to the therapeutic effect of other systemic treatments [6]. Firstly these agents may normalize tumor vasculature and improve delivery of cytotoxic agents. Secondly, they may prevent rapid tumor cell repopulation after cytotoxic drugs. Lastly they may augment the anti-vascular effects of chemotherapy. At present, bevacizumab, a monoclonal antibody directed against VEGF-A, is the only anti-angiogenic agent approved as a treatment for lung cancer [17]. Additional strategies target receptor tyrosine kinases for VEGFR, PDGFR and FGFR, as well as vascular disrupting agents (Table 1). Clinical data for all these of agents are summarized in the following sections.
Table 1
Summary of mechanism of action for anti-angiogenic agents
Drug | VEGF | VEGFR | PDGFR | c-kit | Raf | Flt-3 | RET | Pan-Her | MET | FGFR | EGFR | Src |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
Bevacizumab | ✓ | |||||||||||
Sorafenib | R-2&3 | β | ✓ | ✓ | ✓ | |||||||
Sunitinib | R-1,2&3 | α/β | ✓ | ✓ | ✓ | |||||||
Cediranib | R-1,2&3 | α/β | ✓ | |||||||||
Axitanib | R-1,2&3 | β | ✓ | |||||||||
Motesanib | R-1,2&3 | ✓ | ✓ | ✓ | ||||||||
Linifanib | R-1,2&3 | ✓ | ||||||||||
BMS-690514 | ✓ | ✓ | ||||||||||
MGCD265 | ✓ | ✓ | ||||||||||
Brivanib | R-2 | R-1 | ||||||||||
Vandetanib | R-2&3 | ✓ | ✓ | |||||||||
BIBF1120 | R-1,2&3 | α/β | ✓ | R-1,2&3 | ✓ | |||||||
Pazopanib | R-1,2&3 | α/β | ✓ | R-1,3 | ||||||||
Ramucirumab | R-2 | |||||||||||
Aflibercept | VEGF-A&B |
The Role of Bevacizumab in the Treatment of NSCLC
Bevacizumab in the First-Line Therapy of NSCLC
Bevacizumab is a monoclonal antibody directed towards VEGF-A, and is the only anti-angiogenic agent to date, that has been shown to improve overall survival in lung cancer [ 17]. Toxicity concerns, including venous thromboembolic disease and fatal hemorrhage were observed in the initial randomized phase II trial [18]. This limited further development of bevacizumab in NSCLC to good performance status patients with non-squamous histology and no history of thrombosis, bleeding, gross hemoptysis, or brain metastases.
Five randomized trials have evaluated the addition of bevacizumab to standard platinum-based chemotherapy as first-line therapy for advanced and metastatic NSCLC (Table 2) [17–22]. The Eastern Cooperative Oncology Group (ECOG) 4599 trial randomized patients to carboplatin, paclitaxel with or without bevacizumab 15 mg/kg [17]. Greater efficacy was observed for patients receiving chemotherapy plus bevacizumab, including higher response rates (RR; 35 % vs. 15 %; p < 0.001), longer progression free survival (PFS; 6.2 vs. 4.5 months; hazard ratio [HR], 0.66; 95 % confidence interval [CI], 0.57–0.77) and improved overall survival (OS; 12.3 vs. 10.3 months; HR, 0.79; 95 % CI, 0.67–0.92). The Avastin in Lung Cancer trial (AVAiL) , evaluated the addition of two dose levels of bevacizumab (7.5 and 15 mg/kg) to cisplatin and gemcitabine chemotherapy. Significant improvements were observed in response rates and PFS for patients randomized to either dose of bevacizumab, compared with cisplatin and gemcitabine alone [20]. However, no improvement was observed in OS for patients randomized to bevacizumab 7.5 mg/kg (HR, 0.93; 95 % CI, 0.78–1.11), or 15 mg/kg (HR, 1.03; 95 % CI, 0.86–1.23) [21]. Two additional trials done in Japan and China provide confirmatory data regarding the efficacy of bevacizumab in combination with carboplatin and paclitaxel [19, 22]. Both demonstrate improvements in RR and PFS.
Table 2
Summary of randomized trials evaluating the addition of bevacizumab to systemic therapy for NSCLC
Trial | Design | Intervention | Number | ORR | PFS | OS | QoL |
|---|---|---|---|---|---|---|---|
First-line therapy | |||||||
Johnson [18] | Randomized phase II | Cb+Pac+Bev 7.5 mg/kg Cb+Pac+Bev 15 mg/kg Cb+Pac | 35 32 32 | 31.5 % 28.1 % 18.8 % | 7.4 m 4.3 m 4.2 m | 17.7 m 11.6 m 14.9 m | NA |
ECOG4599 [17] | Phase III | Cb+Pac+Bev 15 mg/kg Cb+Pac | 434 444 | 35 % 15 % P < 0.001 | 6.2 m 4.5 m HR 0.66 (0.57–0.77) | 12.3 m 10.3 m HR 0.79 (0.67–0.92) | NA |
Phase III | Cis-Gem+Bev 15 mg/kg Cis-Gem+Bev 7.5 mg/kg Cis-Gem+Plac | 351 345 347 | 30.5 % 34.1 % 20.1 % | 6.5 m (HR 0.82, p = 0.03) 6.7 m HR (0.79, p = 0.003) 6.1 m | 13.4 m (HR 1.03, 0.86–1.23) 13.6 m (HR 0.93, 0.78–1.11) 13.1 m | NA | |
JO19907 [19] | Randomized Phase II | Cb+Pac+bev 15 mg/kg Cb+Pac | 121 59 | 60.7 % 31 % | 6.9 m 5.9 m (HR 0.61, 0.42–0.89) | 22.8 m 23.4 m (HR 0.99, 0.65–1.50) | NA |
BEYOND [22] | Phase III | Cb+Pac+Bev 15 mg/kg Cb+Pac+Plac | 138 138 | 54.4 % 23.3 % | 9.2 m 6.5 m (HR 0.40, 0.29–0.54) | NA | |
Phase III | Cis+Pem+Bev 7.5 mg/kg then randomized to maintenance Bev 7.5 mg/kg+Pem Bev 7.5 mg/kg | 376 128 125 | 22.7 % | 7.4 m 3.7 m (HR 0.48, 0.35–0.66) | NR 12.8 m (HR 0.75, 0.47–1.19) | EORTC QLQ-30 and LC13. No difference in global QoL. Role function, fatigue and appetite favour bev. Pain favour bev + pem | |
Point Break [35] | Phase III | Cb+Pem+Bev 15 mg/kg → Bev+Pem Cb+Pac+Bev 15 mg/kg → Bev | 472 467 | 34 % 33 % | 6.0 m 5.6 m (HR 0.83, 0.71–0.96) | 13.4 m 12.6 m (HR 1.0, 0.86–1.16) | NA |
Pronounce [36] | Phase III | Cb+Pem → Pem Cb+Pac+Bev 15 mg/kg → Bev | 182 179 | 23.6 % 27.4 % | G4PFS 3.9 m 2.9 m (HR 0.85, 0.70–1.04) | 10.5 m 11.7 m (HR 1.07, 0.83–1.36) | NA |
Second line therapy | |||||||
Herbst [40] | Randomized phase II | Doc/Pem+Bev 15 mg/kg Erlo+Bev 15 mg/kg Doc/Pem+Plac | 40 39 41 | 12.5 % 17.9 % 12.2 % | 4.8 m (HR 0.66, 0.38–1.16) 4.4 m (HR 0.72. 0.42–1.23) 3.0 m | 12.6 m (HR 0.71, 0.41–1.21) 13.7 m (HR 0.78, 0.42–1.23) 8.6 m | NA |
BeTa [39] | Phase III | Erl+Bev 15 mg/kg Erl+Plac | 319 317 | 13 % 6 % | 3.4 m 1.7 m (HR 0.62, 0.52–0.75) | 9.3 m 9.2 m (HR 0.97, 0.80–1.18) | NA |
In all of these trials, bevacizumab was administered as maintenance therapy until disease progression. This strategy has not been formally evaluated, although retrospective review of data from both the ECOG 4599 trial and the US Oncology network suggests that patients continuing bevacizumab until disease progression experienced longer PFS and overall survival [23, 24].
Two meta-analyses of platinum-based chemotherapy with or without bevacizumab both report a significant improvement in OS for patients randomized to receive bevacizumab [25, 26]. Soria et al, report a 10 % reduction in the risk of death from the addition of bevacizumab to chemotherapy (HR 0.90, 95 % CI 0.81–0.99) [25]. Significant improvements were also observed in response rate and PFS [26]. However, the addition of bevacizumab to platinum-based chemotherapy is associated with increased toxicities. In the ECOG 4599 trial, patients randomized to chemotherapy plus bevacizumab experienced more grade 3–5 neutropenia (p = 0.002), thrombocytopenia (p = 0.04), febrile neutropenia (p = 0.02), hyponatremia (p = 0.02), hypertension (p < 0.001), proteinuria (p < 0.001), headache (p = 0.003), rash/desquamation (p = 0.02), and bleeding events (p < 0.001). There were 15 treatment-related deaths, including 5 due to pulmonary hemorrhage [17]. A similar profile of adverse events was observed in the AVAiL trial.
Given the toxicity concerns with bevacizumab, a variety of sub group analyses have been performed on these trials to identify subgroups of patients who derive greater benefit from bevacizumab therapy. In the ECOG 4599 trial, a larger improvement in overall survival was observed in patients with adenocarcinoma histology [27]. The median survival was 14.2 months compared with 10.3 months in the adenocarcinoma subgroup (HR 0.69, 95 %CI 0.58–0.83). Subgroup analyses of Asian patients in the AVAiL trial suggest that the addition of bevacizumab may improve survival in this group of patients [28]. Biomarkers including serum VEGF, intracellular adhesion molecule (ICAM), βFGF have all been evaluated as predictive biomarkers for bevacizumab. While ICAM was prognostic for improved survival and both ICAM and VEGF were predictive for response rates, no biomarker to date has been shown to predict a differential effect on OS for patients receiving bevacizumab. The onset of hypertension, a known side effect of bevacizumab, has also been evaluated as a predictive biomarker of efficacy [29]. However, a combined analysis of seven trials including disease sites other than lung cancer, did not support hypertension as a predictive biomarker. Therefore none of the biomarkers evaluated to date, are predictive for an improvement in overall survival from bevacizumab [30]. Therefore, it is not possible to further define subgroups of patients to receive bevacizumab therapy.
Competing therapeutic strategies to bevacizumab have emerged over recent years. Maintenance therapy with pemetrexed following initial platinum-doublet chemotherapy has been shown to improve survival in advanced NSCLC [31, 32]. Therefore, additional trials have tried to evaluate the role of pemetrexed, in bevacizumab eligible patients. Two trials have incorporated maintenance pemetrexed therapy in patients receiving bevacizumab. The AVAPERL trial randomized patients to maintenance therapy with bevacizumab alone (Table 2), or in combination with pemetrexed, following induction therapy with cisplatin, pemetrexed plus bevacizumab [33]. Three hundred and seventy six patients were registered and 253 randomized to maintenance therapy. PFS, the primary outcome, was prolonged in patients receiving maintenance bevacizumab plus pemetrexed compared with bevacizumab alone (7.4 m vs. 3.7 m HR 0.48, 95 %CI 0.35–0.66). AVAPERL failed to show a significant difference in overall survival as a secondary endpoint (HR 0.75, 95 %CI 0.47–1.19). Global quality of life (QoL), assessed by the EORTC QLQ-30, was similar between the two arms, although role function, fatigue and appetite favoured the combination of bevacizumab and pemetrexed [34]. Similarly, the Point Break trial evaluated the addition of pemetrexed to maintenance bevacizumab [35]. Patients were randomized to carboplatin, paclitaxel, bevacizumab then maintenance bevacizumab, versus carboplatin, pemetrexed, bevacizumab then maintenance pemetrexed plus bevacizumab. There was a modest improvement in PFS (6 m vs. 5.6 m, HR 0.83, 95 %CI 0.71–0.96), but no improvement in overall survival (13.4 m vs. 12.6 m, HR 1.0, 95 %CI 0.86–1.16). Maintenance therapy with pemetrexed in addition to bevacizumab should not be considered the standard of care at this time in the absence of improved overall survival.
One additional trial (Pronounce) compared carboplatin, paclitaxel plus bevacizumab with a non-bevacizumab regimen of carboplatin, pemetrexed then maintenance pemetrexed [36]. The primary outcome was a composite of progression free survival without grade 4 toxicity. Similar response rates, PFS and overall survival were observed between the two arms, suggesting that bevacizumab may not add to the efficacy of more effective chemotherapy. Currently, bevacizumab is widely used in combination with platinum-based chemotherapy and has been incorporated into NSCLC treatment algorithms [37]. It does have significant incremental toxicity and data from the Pronounce trial suggest that cisplatin or carboplatin plus pemetrexed followed by maintenance pemetrexed provides an alternative to bevacizumab based therapy.
Bevacizumab in the Second Line Therapy of Advanced NSCLC
There has been some interest in continuation of bevacizumab beyond progression, however, there are no data from randomized trials to support this. There is an ongoing trial (AVaALL) evaluating this strategy [38]. However, two trials have evaluated the addition of bevacizumab to erlotinib in the second-line setting [39, 40]. Herbst et al. [40], conducted a randomized phase II trial comparing the addition of bevacizumab to either second-line chemotherapy, or erlotinib. Response rates were highest with the combination of erlotinib plus bevacizumab (Table 2) and improvements in PFS and OS were seen with both bevacizumab containing arms. Subsequently, the phase III BeTa trial randomized patients to erlotinib plus bevacizumab versus erlotinib alone [39]. Significant improvements were observed in RR (13 % vs. 6 %) and PFS (3.4 m vs. 1.7 m, HR 0.62, 95 %CI 0.52–0.75). However, there was no improvement in overall survival (9.3 m vs. 9.2 m, HR 0.97, 95 %CI 0.80–1.18). As a result, there are no data currently supporting the use of bevacizumab as second-line therapy in combination with either erlotinib or chemotherapy.
Other Intravenous Anti-Angiogenic Compounds Evaluated in NSCLC
Ramucirumab
Ramucirumab is an IGG1 humanized monoclonal antibody targeting the VEGFR-2 receptor. It is being evaluated in a number of disease sites including NSCLC. A phase II trial of ramucirumab in combination with carboplatin and paclitaxel was reported at the American Society of Clinical Oncology meeting in 2010 (Table 3) [41]. Data were reported on 31 patients with NSCLC (all histologies were allowed). There were 10 responses in 15 evaluable patients (RR 67 %). Detailed toxicity information was not available.
Table 3
Other intravenous anti-angiogenic compounds that have been evaluated in NSCLC
Trial | Design | Intervention | Number | ORR | PFS | OS | QoL |
|---|---|---|---|---|---|---|---|
First-line therapy | |||||||
Ramucirumab | |||||||
Camidge [41] | Phase I | Cb+Pac+Ram | 22 evaluable | 10/15 (67 %) | 5.7 m (95 % CI 5.62–5.75) | NR | NA |
Doebele [42] | Randomized phase II | Cis/Cb+Pem+Ram 10 mg/kg → Pem+Ram Cis/Cb+Pem → Pem | 69 71 | 49.3 % 38 % | 7.2 m (HR 0.75, 90 % CI 0.55–1.03) 5.6 m | 13.9 m (HR 0.83, 90 % CI 0.56–1.22) 10.4 m | NA |
Aflibercept | |||||||
Chen [44] | Phase II | Cis+Pem+Aflib | 42 | 26.3 % | 5 m (95 % CI 4.3–7.1 m) | NR | Trial stopped early because 3 cases RPLS |
Vadimezan | |||||||
McKeage [47] | Randomized phase II | Cb+Pac+Vad → Vad Cb+Pac | 37 36 | 34.4 % 29 % | 5.4 m (HR 0.86, 0.5–1.4) 4.4 m | 14.0 m (HR 0.73, 0.39–1.38) 8.8 m | NA |
ATTRACT-1 [48] | Phase III | Cb+Pac+Vad → Vad Cb+Pac+Plac → Plac | 649 650 | 24.7 % 24.6 % | 5.5 m (HR 1.04, 0.–91 1.19) 5.5 m | 13.4 m (HR 1.01, 0.85–1.19) 12.7 m | EORTC QLQ30 – no differences observed |
Fosbretabulin | |||||||
FALCON [110] | Randomized phase II | Carb+Pac+Bev+Fos Carb+Pac+Bev | 31 29 | 55 % 38 % | 8.6 m (HR 0.89, 0.46–1.73) 8.8 m | – | NA |
Second-line therapy | |||||||
VITAL [45] | Phase III | Doc+Aflib Doc+Plac | 456 457 | 23.3 % 8.9 % | 5.2 m (HR 0.82, 0.72–0.94) 4.1 m | 10.1 m (HR 1.01, 0.87–1.17) 10.4 m | LCSS – no differences observed |
Doebele et al, reported the results of a randomized phase II trial of cisplatin or carboplatin in combination with pemetrexed, with or without ramucirumab for four to six cycles [42]. Pemetrexed was continued as maintenance therapy either alone or with ramucirumab. A total of 140 patients were randomized. The addition of ramucirumab appeared to improve the efficacy of a platinum agent plus pemetrexed. Higher response rates were observed (49.3 % vs. 38 %). There were trends towards improved PFS (HR 0.75, 90 %CI 0.55–1.03) and OS (HR 0.83, 90 %CI 0.56–1.22), although these did not achieve statistical significance. Patients randomized to ramucirumab experienced more grade 3 neutropenia, anemia, thrombocytopenia, nausea, fatigue, hypertension, and back pain.
At this point ramucirumab remains an investigational agent with some promise. There are ongoing randomized phase II trials in both first and second-line therapy for NSCLC.
Aflibercept
Aflibercept is a human fusion protein designed to block biding of VEGF-A and VEGF-B, as well as human placental growth factor (commonly referred to as VEGF-trap). It has evidence of minor single agent activity in heavily pretreated patients [43]. A phase II trial of first-line therapy with aflibercept in combination with cisplatin and pemetrexed showed modest activity [44]. The RR was 26.3 % with median PFS of 5 months. However, the trial was stopped early because of three cases of reversible posterior leukoencephalopahy syndrome (RPLS). A randomized phase III trial of docetaxel plus or minus aflibercept as second-line therapy, showed some evidence of activity for the addition of aflibercept [45]. The addition of aflibercept to docetaxel improved RR (23.3 % vs. 8.9 %, p < 0.001) and PFS (HR 0.82, 95 %CI 0.72–0.94). However, the study failed to achieve its primary outcome of improved overall survival. In addition there were no improvements in quality of life as measured by the Lung Cancer Symptom Scale (LCSS). There are multiple ongoing trials of aflibercept in other cancers. However, data to date do not support the use of aflibercept in NSCLC.
Vadimezan
Vadimezan is another VEGF-Trap compound [46]. Activity was observed in early phase clinical trials. A randomized phase II trial of carboplatin and paclitaxel plus/or minus vadimezan suggested that vadimezan may improve the effectiveness of platinum-based chemotherapy [47]. However, a phase III trial of the same regimen failed to improve on treatment outcomes [48]. There were no significant differences in response rates, PFS and overall survival. Additionally, quality of life between the two groups was similar. Therefore, vadimezan does not improve the therapeutic options for patients with advanced NSCLC.
Small Molecule Oral Anti-Angiogenic Compounds in the First-Line Therapy of NSCLC
There are multiple small molecule anti-angiogenic compounds that have been evaluated in combination with platinum-based chemotherapy, in the first-line therapy for NSCLC (Table 4). These agents inhibit one or more angiogenic pathways (VEGFR, PDGFR and/or FGFR), in addition to other off target receptors (Table 1). To date, despite promising preliminary data, none of these compounds have improved the therapeutic efficacy of platinum-based chemotherapy alone. Drug related toxicities of some agents preclude the administration of full doses when combined with chemotherapy. Incremental toxicity was seen in all the trials and in some cases appears to increase the risk of death. As such there are no data to support the addition of an oral anti-angiogenic agent to platinum-based chemotherapy.
Table 4
Summary of trials of oral anti-angiogenic agents in combination with platinum-based chemotherapy as first-line therapy for NSCLC
Trial | Design | Intervention | Number | ORR | PFS | OS | QoL |
|---|---|---|---|---|---|---|---|
Sorafenib | |||||||
ESCAPE [53] | Phase III | Cb+Pac+Soraf Cb+Pac+Plac | 464 462 | 27.4 % 24 % | 4.6 m (HR 0.99, 0.84–1.16) 5.4 m | 10.7 m (HR 1.15, 0.94–1.41) 10.3 m | NA Trial stopped early for futility |
NEXUS [52] | Phase III | Cis+Gem+Soraf Cis+Gem+Plac | 385 387 | 28 % 26 % | 6.0 m (HR 0.83, 0.71–0.97) 5.5 m | 12.4 m (HR 0.98, 0.83–1.16) 12.5 m | NA |
Cediranib | |||||||
NCIC IND 171 [55] | Phase I | Cb+Pac+Ced | 20 | 45 % (23–68 %) | 7.6 m | NA | |
BR24 [56] | Randomized phase II | Cb+Pac+Ced 30 mg Cb+Pac+Plac | 126 125 | 38 % 16 % | 5.6 m (HR 0.77, 0.56–1.08) 5.0 m | HR 0.78 (0.57–1.06) | EORTC QLQ30 and LCS13 – not reported |
BR29 [57] | Phase III | Cb+Pac+Ced 20 mg Cb+Pac+Plac | 153 153 | 52 % 34 % | 5.5 m (HR 0.91, 0.71–1.18) 5.5 m | 12.2 m (HR 0.94, 0.69–1.30) 12.1 m | EORTC QLQ30 and LCS13 – not reported |
NCCTG528 [58] | Randomized phase II | Cb+Gem+Ced 30 mg Cb+Gem+Plac | 60 31 | 19 % 20 % | 6.3 m (HR 0.69, 0.43–1.09) 4.5 m | 12 m (HR 0.66, 0.41–1.08) 9.9 m | NA |
Motesanib | |||||||
NCT00369070 [60] | Randomized phase II | Cb+Pac+Mot 125 mg daily Cb+Pac+Mot 75 mg bid Cb+Pac+Bev 15 mg/kg | 61 62 63 | 30 % 23 % 37 % | 7.7 m (HR 1.14, 0.73–1.76) 5.8 m (HR 1.22, 0.80–1.85) 8.3 m | 14.0 m (HR 1.05, 0.67–1.63) 12.8 m (HR 1.18, 0.76–1.83) 14.0 m | NA |
MONET-1 [61] | Phase III | Cb+Pac+Mot Cb+Pac+Plac | 541 549 | 40 % 26 % | 5.6 m (HR 0.79, 0.68–0.90) 5.4 m | 13 m (HR 0.90, 0.78–1.04) 11 m | NA |
Vandetanib | |||||||
Heymach [64] | Randomized phase II | Cb+Pac+Vandet Vandet Cb+Pac | 56 73 52 | 32 % 7 % 25 % | 24w (HR 0.76, p = 0.098) 11.5w (HR 1.26, p = 0.86) 23w | 10.2 m (HR 1.15, p = 0.738) 10.2 m (HR 1.09,p = 0.651) 12.6 m | NA |
PrE0501 [63] | Randomized phase II | Cb+Doc+Vandet → Vandet Plac | 162 total | 4.5 m (p = 0.07) 4.2 m | 9.8 m (p = 0.68) 9.4 m | NA | |
Pazopanib | |||||||
Scagliotti [66] | Randomized phase II | Pem+Pazop Cis+Pem | 71 35 | 23 % 34 % | 25w (HR 0.75, 0.43–1.28) 22.9w | HR 1.22 (0.64–2.33) | Trial stopped early because of increased treatment related deaths |
Axitanib | |||||||
Belani [68] | Randomized phase II | Cis+Pem+Axit D1–21 Cis+Pem+Axit D2–19 Cis+Pem | 55 58 57 | 45.5 % 39.7 % 26.3 % | 8.0 m (HR 0.89, 0.56–1.4) 7.9 m (HR 1.02, 0.64–1.62) 7.1 m | 16.6 m (HR 1.08, 0.66–1.760) 14.7 m (HR 1.39, 0.87–2.22) 15.9 m | NA |
Sorafenib
Sorafenib is a multitargeted TKI active against VEGFR, PDGFR, Raf, c-Kit and FLT-3 [49]. Preliminary data from heavily pretreated NSCLC patients showed disease stabilization in 59 % of patients [50]. As a result, sorafenib underwent further development in NSCLC. A phase I/II trial of sorafenib in combination with standard doses of carboplatin and paclitaxel demonstrated sorafenib could be administered in full doses with chemotherapy [51]. Subsequently, two phase III trials were conducted [52, 53]. The ESCAPE trial randomized 926 patients to carboplatin and paclitaxel plus sorafenib or placebo [53]. Patients were all good performance status (ECOG 0-1) and all NSCLC histologies were included in the trial. The trial was stopped early following an interim analysis that met criteria for futility. At the time of the final analysis, no differences were observed in OS between the two groups (median OS 10.7 m vs. 10.3 m, HR 1.15, 95 %CI 0.94–1.41). Additionally, there were no differences observed in the secondary outcomes (RR or PFS) and quality of life was not assessed in the trial. Interestingly a planned analysis according to histology demonstrated that patients with squamous cancer randomized to sorafenib had worse OS than patients in the placebo group (8.9 m vs. 13.6 m, HR 1.85, 95 %CI 1.22–2.81). Patients randomized to sorafenib experienced more thrombocytopenia, rash / desquamation, hand-foot reaction, hypertension and pruritis than in the placebo group. However, patients with squamous cancer did not experience incrementally worse toxicity.
A similarly designed trial (NEXUS), evaluated the addition of sorafenib or placebo, to cisplatin and gemcitabine in 772 patients [52]. Recruitment of patients with squamous cancer was halted following analysis of the ESCAPE trial. Data have been reported on the non-squamous histology population. No improvement in OS was observed for patients randomized to sorafenib (12.4 m vs. 12.5 m, HR 0.98, 95 %CI 0.83–1.16). A similar toxicity profile was observed to that seen in the ESCAPE trial. In summary there is no evidence to support the addition of sorafenib to first-line platinum-based chemotherapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree